Amelogenins are enamel matrix proteins currently used to treat bone defects in periodontal surgery. Recent studies have highlighted the relevance of amelogenin-derived peptides, named LRAP, TRAP, SP, and C11, in bone tissue engineering. Interestingly, these peptides seem to maintain or even improve the biological activity of the full-length protein, which has received attention in the field of bone regeneration.
1. Introduction
A wide range of pathological phenomena can be directly or indirectly responsible for skeletal tissue loss. Many of the generated defects, known as critical size defects (CSDs), do not heal spontaneously. The best option to treat CSDs is an autologous bone graft that possesses the three main features needed in bone regenerative medicine: osteogenic, osteoinductive, and osteoconductive properties. Unfortunately, autologous bone graft is rarely used due to a number of drawbacks, such as the requirement of a second surgical procedure with serious risks of infection at the donor site and the generation of significant pain.
Regenerative medicine and tissue engineering offer alternative strategies for the treatment of CSDs. In most of the approaches proposed, molecules capable of stimulating cell migration, recruitment, proliferation, and differentiation, as well as biomineralization, play a pivotal role in the formation of de novo bone tissue. Platelet-derived growth factors, insulin-like growth factors, transforming growth factors, and bone morphogenic proteins are examples of biomolecules investigated so far [1]. However, several efforts are still ongoing to individuate effective and safe bone morphogenic biomolecules. Among the biological macromolecules under investigation, amelogenins (AMG) represent an extremely interesting family of proteins with the above-mentioned characteristics for which the bone morphogenic properties are still matter of debate.
AMG-based preparations were first proposed in the dermatological field for the treatment of burns and, only later, in dentistry. The first AMG formulation marketed for periodontal tissue regeneration procedures was Emdogain ® . The product contains a mixture of animal enamel matrix derivatives embedded in an alginate propylene glycol hydrogel. After 20 years, the use of Emdogain ® in periodontal regeneration procedures has shown a statistically significant improvement in the recovery of the periodontal ligament, cement, and alveolar bone [2][3].
Although for over four decades, AMG was considered a specific enamel protein expressed in periodontal tissues, such as cementoblasts, periodontal ligament (PDL) cells, or Hertwig’s epithelial root sheath (HERS) [3][4][5][6][4,5,6,7], its expression has also been reported, at a lower level, in non-dental cell types, such as stem cells, bone cells, brain, and other soft tissue [7][8][9][8,9,10]. Of interest are some observations suggesting that specific AMG splicing products may function as epithelial-mesenchymal or mesenchymal-mesenchymal signaling molecules [10][11][12][13][11,12,13,14]. In the late 1960s and early 1970s, two articles showed the osteoinductive potential of decalcified enamel and dentin extracts in ectopic sites [14][15][15,16]. This phenomenon was attributed to the presence of peptides with chondro-/osteoinduction properties derived from AMG gene splicing [16][17]. AMG-derived peptides are formed by alternative splicing or proteolytic cleavage of the ~20 kDa full-length protein [17][18][18,19].
2. LRAP
The Leucine-Rich Amelogenin Peptide (LRAP) is a 59-residue natural splice-variant of AMG
[19][127]. The spliced mRNA encodes a 79-residue peptide referred to as Pro-LRAP that undergoes the telopeptide proteolytic cleavage between residue 167 and 168 by MMP-20 leading to the mature LRAP. This peptide was isolated and purified from secretory enamel matrix by Fincham and coworkers in 1981
[20][128], but only later was it recognized as an osteogenesis-inducing factor
[16][17]. The 59-residue peptide is composed of the first 33 and last 26 AMG residues and is a naturally occurring product of alternative splicing of the primary mRNA transcript, with the N- and C-terminal charged regions of the full-length protein.
The secondary structure is mainly constituted by random coils in the C-terminus and N-terminus of the protein
[21][22][23][129,130,131], even though numerical simulations suggest the presence of partially helical regions at the C-terminus (aa 48–55) and the N-terminus (aa 12–17)
[23][131].
Yamazaki and colleagues have investigated the role of protein phosphorylation on LRAP secondary structure in the presence of hydroxyapatite and amorphous calcium phosphate. They showed that LRAP(-P) mainly consist of random coil and PPII helix or β-sheet structure, while LRAP(+P) exhibits more β-sheet and α-helix, with little random coil. With the addition of Ca
2+, the random coil content increased in LRAP(-P), while LRAP(+P) exhibited a decrease in α-helix components. Incubation of LRAP(-P) with hydroxyapatite or amorphous calcium phosphate resulted in comparable increases in β-sheet structure. Notably, LRAP(+P) secondary structure was more affected by amorphous calcium phosphate than by hydroxyapatite, mainly showing an increase in β-sheet structure
[24][132].
LRAP seems flexible enough to have several possible tertiary conformations.
Concerning quaternary structure, LRAP is primarily a monomer over a wide range of concentrations, pH values, salt concentrations, and in the presence of calcium. However, Ma et al. showed a hierarchical LRAP self-assembling with the formation of nanospheres, nanorods, associated nanospheres (nano strings), gel-like precipitations, and amyloid-like structures. LRAP amyloid-like supramolecular structures are assemblies containing a consistent rigid β-sheet secondary structure (residues 12–27) that seems to be essential for amyloidogenic aggregation
[25][133]. LRAP monomers are the dominant species in solutions at pH values higher than the isoelectric point, but the solutions also contain a relatively low concentration of oligomeric species (0–16%). The differences in quaternary structures between LRAP and AMG may show which domains are important in the formation of supramolecular structures. Indeed, the missing central region is necessary for nanosphere formation since it promotes oligomer–oligomer binding.
Several studies have tried to elucidate the physiological role of LRAP in enamel formation. Its involvement in cell signaling
[7][26][27][28][29][8,24,34,35,134], in the regulation of the calcium phosphate mineralization kinetics, and the morphology of formed crystals has been reported
[30][24][31][25,132,135]. LRAP shares many common properties with the full-length AMG in the regulation of mineral formation in vitro: it forms nanospheres
[30][32][33][25,136,137] and binds hydroxyapatite
[34][35][138,139]. In vitro experiments using non-phosphorylated recombinant human LRAP and recombinant human AMG (rH174) showed that they have the same ability to bind calcium (i.e. 4 to 6 calcium ions per molecule), although the calcium affinity constant for the LRAP was greater than that observed for AMG
[36][140].
3. TRAP
Tyrosine-Rich Amelogenin Peptide (TRAP) is a 5.1 kDa isoform of EMD generated from the full-length AMG through proteolytic cleavage. The amino acid sequence is the following: MPLPPHPGHPGYINFSYEVLTPLKWYQNMIRHPYTSYGYEPMGGW.
The biological role of TRAP in periodontal cells is still matter of debate. In fact, it does not seem to have any effect, not even a suppressive effect, on alveolar bone or the periodontal ligament
[37][38][28,84]. On the other hand, it was reported that chemically synthesized TRAP stimulates angiogenic differentiation of human periodontal ligament stem cells
[39][40][41][29,30,160] and angiogenesis in human gingival fibroblasts
[42][161].
The angiogenic property
[43][44][162,163] and expression of angiogenesis-related proteins in endothelial cells (ECs)
[45][46][47][48][88,164,165,166] have been widely documented in several in vitro and in vivo studies. However, EMD-derived fraction containing TRAP seems more active than TRAP in stimulating angiogenesis
[37][28]. A plausible explanation of this biological effect was given by Jonke et al. in 2016
[42][161]. TRAP, isolated from EMD or chemically synthesized, upregulates the expression of adhesion molecules as ICAM-1 and E-selectin, localized on the endothelial cell surface
[49][50][167,168]; VEGF, that plays a central role in angiogenesis
[51][169]; kinase insert domain receptor (KDR), localized on the endothelial cell surface, with an important role in endothelial cell differentiation; and the FMS-like tyrosine kinase receptors 1 (FLT-1)
[52][170].
Von Willebrand factor (a multimeric glycoprotein known for its contribution to the hemostatic process) upregulation by TRAP was reported for the first time by Amin and colleagues in 2014, and in 2016 by Jonke at al.
[39][42][29,161]. These authors suggest that TRAP might improve interaction between different cell types and promote VEGF release by resident fibroblasts and VEGF response by endothelial cells during the process of wound healing. Interestingly, TRAP seems to be able also to increase the expression of VEGFR2, an early endothelial marker gene (a tyrosine kinase receptor for the VEGF ligand) on endothelial cells, together with the late genes Tie-1 and Tie-2, two tyrosine kinase receptors for angiopoietin that are exclusively expressed by endothelial cells
[53][171], and VE-cadherin, an endothelial cell adhesion molecule in human periodontal cells. The angiogenic effect was also demonstrated by using the chicken embryo chorioallantoic membrane assay
[39][29]. The proliferation/viability of endothelial cells was significantly decreased after treatment with TRAP, peptides with either 43 or 45 aminoacidic residues separated and purified from EMD, and synthetic TRAP at a concentration of 100 μg/ml
[42][161].
The low-molecular-weight EMD fraction, containing TRAP, has an osteoblastic effect on PDL fibroblasts
[41][160]. The authors have found increased
RUNX2, OPN, OCN, BSP gene expression levels and an increased alkaline phosphatase activity.
In adult primary human articular cartilage cells (HACs), TRAP seems to suppress hypertrophic mineralization and concomitantly promotes chondrogenic differentiation through both early and late chondrogenic gene induction (i.e., SOX9,
COL2A1, and ACAN). These results were observed when cells were cultured in chondrogenic conditions supplemented with TRAP (10 μg/mL) but not in control medium (
Figure 14)
[54][38]. These results are in agreement with previous reports in which they demonstrated that TRAP suppresses bone-forming activity through Smad6-mediated
RUNX2 inhibition
[37][28]. In the same year, Tanimoto and colleagues showed no significant effects on mineralization and expression of osteogenic markers of TRAP on human periodontal ligament cells (HPDLs)
[55][124].
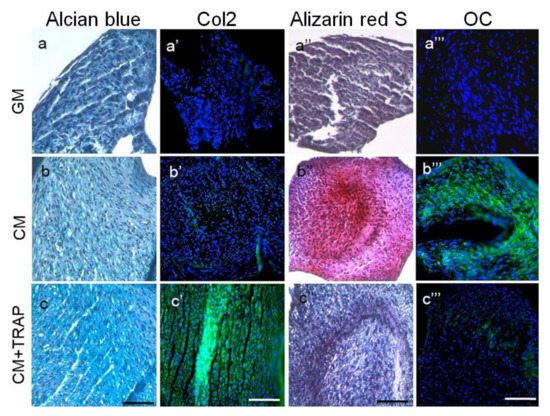
Figure 14. Histology indicates that 10 μg/ml TRAP promotes chondrogenic differentiation and suppresses hypertrophic mineralization. Labels on individual panels refer to culture media type: (
a–
a'''’’’) GM; (
b–
b'''’’’) CM; (
c–
c'''’’’) CM+TRAP. Note the intense bright blue staining of TRAP-treated cell pellets stained with alcian blue (
c, indicative of glycosaminoglycans present in the ECM) and the corresponding lack of Alizarin red staining (
c''’’, indicative of minimal calcium deposition). Note also the green fluorescent staining of TRAP-treated cell pellets immuno-stained with Col2 (
c'’) and the corresponding lack of OCN staining (
c'''’’’). Alcian blue and alizarin red sections were counter-stained with Harris Haematoxylin (purple nuclei); Col2 and OCN sections were counter-stained with Hoechst dye (blue nuclei). Scale bar 100 μm. (Reproduced from
[54][38], an open access article distributed under the terms of the Creative Commons CC BY license.)
4. SP (Synthetic Peptide)
The bioactivity of EMD varies from batch to batch and has been reported to be antigenic and to induce the production of anti-EMD antibodies in the host. To overcome these issues, Kim et al. analyzed the active sites for eosinophilic round bodies (ERBs) binding in EMD-associated proteins using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. They identified a 7 amino acid sequence (WYQNMIR) in AMG that corresponds to a portion of the AMG exon 5. This sequence, named SP, has a molecular mass of 1118 Da, and it is less likely than EMD to elicit an immunological response
[56][31].
The use of a synthetic peptide would allow one to avoid the use animal derivatives and would be suitable in terms of safety and reproducibility. Scientific evidence on its potential use in tissue engineering and regenerative medicine appeared in the literature only in 2009
(Table 2).
Periodontal ligament fibroblast treated with SP had an increased expression of genes related to osteogenesis, such as BMP receptor type 1 A, BMP4, osteonectin, BMP receptor type1 B, osteocalcin, as well as ALP activity, and intracellular calcium deposition with a parallel decreased expression of fibroblast growth factor receptor-like protein 1
[57][58][39,42].
These data indicate that SP is effective in periodontal tissue regeneration, suggesting that it may convert human periodontal ligament fibroblasts to bone-forming cells and act as a growth factor. On rat bone marrow cell cultures, SP increases cell proliferation, adhesion, and chemotaxis, reporting significantly higher alkaline phosphatase activity and Ca
2+ deposition after 7 and 14 days of treatment
[59][41]. Kato and colleagues investigated SP’s effect on human periodontal ligament stem cells (PDLSCs), concluding that it can enhance osteoblastic differentiation at early stages of differentiation and can be effective in the initial stage of periodontal tissue regeneration. The authors showed that cell proliferation is significantly increased in the presence of SP in both normal and osteogenic medium. In SP-supplemented osteogenic medium, the expression of osteonectin and osteocalcin mRNA, ALP activity, the number and size of calcified nodules, mineralization, and osteocalcin production were significantly higher than non-supplemented osteogenic medium (
Figure 25A)
[60][43].
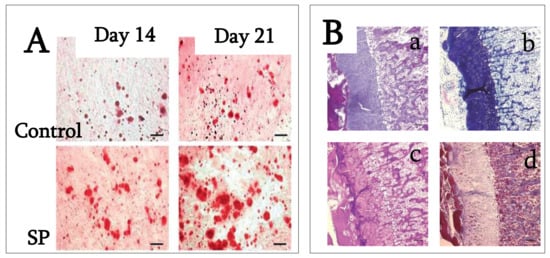
Figure 25. (
A) PDLSC cultures were stained with alizarin red S after 14 and 21 days of cultivation in osteogenic medium with and without 100 ng/mL SP. Calcium deposition on days 14 and 21 was higher in the presence of SP than in its absence. Bar = 100 mm. (Reproduced with permission from
[60][43]). (
B) Endochondral ossification and bone formation are observed in the backs of rats 14 days after injection in rats injected with 15 mg/mL concentration of synthetic peptide (
a): hematoxylin and eosin stain). Metachromasia is demonstrated at cartilage tissue with toluidine blue (
b). Both cartilage and bone tissue are positive for PAS (
c), but negative for Masson trichrome (
d). (
a–
d, original mag. ×5; bar: 0.1 mm; reproduced from
[61][40].)
Thus, SP apparently promotes the mineralization of extracellular matrix on PDLSCs, suggesting that SP promotes PDLSC proliferation, the expression of mineralization markers, and the formation of calcified nodules highlighting the potential of SP in periodontal tissue regeneration. In 2014, Katayama et al. synthesized the same oligopeptide derived from EMD to evaluate its contribution to periodontal tissue regeneration. They investigated the SP effects on cell proliferation and osteoblastic differentiation of human mesenchymal stem cells (MSCs). SP (0 to 1000 ng/mL) promoted cell proliferation, osteoblast differentiation, and matrix mineralization, probably through the ERK signaling pathway
[62][44].
These results indicate that SP would be useful for periodontal and bone tissue regeneration because the promotion of MSCs proliferation and differentiation is described as being essential in these processes. Moreover, SP is unlikely to be antigenic and it can be produced synthetically. However, the molecular mechanisms by which SP acts on cells are not yet clearly understood and must be further investigated. Only one paper has investigated SP’s ability to induce the extracellular matrix mineralization and bone tissue formation in animal models. SP seems to produce heterotopic ossification in rats when injected under the skin, with endochondral ossification and bone formation within 14 days from the injection (
Figure 25B)
[61][40].
5. C11 (Amelogenin C Peptide, AMG-CP)
A variety of studies suggest that the C-terminal tail of AMG (C11) plays an important role in enamel biomineralization
[63][172]. Zhu et al., using AMG mutated variants (P156T and P164T), demonstrated that the substitution of proline with threonine at position 156 or 164 displayed a significantly lower affinity to HAP, suggesting that these 2 C-terminal prolines are important for optimal adsorption of AMG protein to HAP. The prolines appear to be essential conformation determinants that alter the accessibility of AMG C-terminus to apatite, which is related to the growth of apatite crystals and enamel development
[64][173].
It is noteworthy that prolines are found adjacent and upstream to all identified MMP-20 cleavage sites in the AMG sequence
[65][174]. Proline is the only cyclic amino acid with a pyrrolidine ring that restricts the conformation range of adjacent residues. The regularly spaced prolines are presumably important in maintaining the extended chain conformation of proteins. The proline residues in the AMG C-terminus are highly conserved across many species, suggesting a functional role for the initial processing of AMG and AMG–mineral interactions.
C11 stimulates the proliferation of human cementoblast-like cell line
[66][175], hAD-MSCs, and hBM-MSCs
[67][68][32,33], enhancing the phosphorylated ERK1/2 signaling in both cell lines. These effects are inhibited by an anti-LAMP-1 antibody, a condition that further demonstrates the importance of proteins associated with the endosomal/lysosomal membrane in the signal regulation of these peptides
[68][66][33,175].
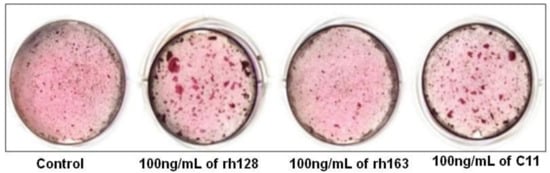
Regarding C11’s biological role in osteogenic differentiation, the available data indicate that human cementoblast osteogenic differentiation was significantly enhanced by rh128, AMG fragments lacking the N-terminus, and C11, while rh163, AMG fragments lacking the C-terminus, had no significant effect. This indicates the possible utility of C11 in periodontal tissue regeneration (
Figure 36)
[69][46]. On the contrary, Awada et al., by using similar C11 concentrations, have found an increased proliferation of mouse MC3T3-E1 cells, but no substantial effects on osteogenic differentiation
[70][45].
Figure 36. Effect of amelogenin fragment treatment on the mineralization activity of HCEM cells during osteogenic differentiation. Intensity of alizarin red staining in HCEM cells increased following treatment with rh128 or C11 peptide, but there was no obvious difference following treatment with rh163. (Reproduced with permission from
[69][46].)
Overall, these studies suggest the need of future investigations to elucidate C11 molecular mechanisms, by considering also possible differences due to different experimental protocols.