Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alvaro Compañ-Bertomeu | + 3193 word(s) | 3193 | 2021-09-16 08:23:55 | | | |
| 2 | Jessie Wu | Meta information modification | 3193 | 2021-10-08 03:15:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Compañ-Bertomeu, A.; Alcaraz, M.J.; Guillén Salazar, I. Extracellular Vesicles as Novel Treatments. Encyclopedia. Available online: https://encyclopedia.pub/entry/14816 (accessed on 07 February 2026).
Compañ-Bertomeu A, Alcaraz MJ, Guillén Salazar I. Extracellular Vesicles as Novel Treatments. Encyclopedia. Available at: https://encyclopedia.pub/entry/14816. Accessed February 07, 2026.
Compañ-Bertomeu, Alvaro, Maria José Alcaraz, Isabel Guillén Salazar. "Extracellular Vesicles as Novel Treatments" Encyclopedia, https://encyclopedia.pub/entry/14816 (accessed February 07, 2026).
Compañ-Bertomeu, A., Alcaraz, M.J., & Guillén Salazar, I. (2021, October 02). Extracellular Vesicles as Novel Treatments. In Encyclopedia. https://encyclopedia.pub/entry/14816
Compañ-Bertomeu, Alvaro, et al. "Extracellular Vesicles as Novel Treatments." Encyclopedia. Web. 02 October, 2021.
Copy Citation
Mesenchymal stem/stromal cells (MSCs) represent a promising therapy for musculoskeletal diseases. There is compelling evidence indicating that MSC effects are mainly mediated by paracrine mechanisms and in particular by the secretion of extracellular vesicles (EVs). Many studies have thus suggested that EVs may be an alternative to cell therapy with MSCs in tissue repair.
mesenchymal stem cells
extracellular vesicles
immunoregulation
bone diseases
osteoarthritis
1. Introduction
Musculoskeletal disorders and injuries are the leading cause of disability worldwide with a high prevalence across the life-course. People with multi-morbidity are particularly vulnerable especially in the context of an aging population. These disorders are painful and lead to mobility limitation and early retirement with a high economic and social impact. They include a wide range of conditions such as osteopenia, osteoporosis, fractures, osteoarthritis (OA), rheumatoid arthritis (RA), sarcopenia and so forth [1].
Transplantation of mesenchymal stem/stromal cells (MSCs) has been recognized in recent years as a promising therapy for musculoskeletal diseases. Preclinical models have provided evidence that MSCs have potential applications in these conditions due to their regenerative and immunomodulatory properties. MSC exhibit a variety of trophic activities relevant to musculoskeletal therapy (reviewed in Reference [2]). The efficacy of MSC treatment has been demonstrated in animal models and clinical studies of RA, OA and cartilage repair [3][4][5][6][7], as well as in bone [8][9], tendon [10] and skeletal muscle [11] regeneration. Although more research is needed including controlled clinical studies with long-term follow-up, these results open the possibility of improving current therapies.
A wide range of evidence has demonstrated that paracrine mechanisms are main components of MSC regenerative effects. MSCs respond to stimuli present in the local microenvironment by secreting a variety of bioactive molecules. Accordingly, it is possible to modulate the composition of the MSC secretome by cellular pre-conditioning during culture, thus maximizing their potential for therapeutic applications (reviewed in Reference [12]). The conditioned medium (CM) from MSCs contains multiple factors that may cooperate to induce a repair response. Besides extracellular vesicles (EVs), MSCs can secrete a wide range of molecules such as purines, bone morphogenetic proteins (BMPs), CD274, C-C motif chemokine ligand (CCL)-2, connexin 43, indoleamine 2,3-dioxygenase, prostaglandin (PG)E2, interleukin(IL)-6, IL-10, NO, heme oxygenase-1, tumor necrosis factor-inducible gene-6 (TSG-6), leukemia inhibitory factor (LIF), CD95/CD95 ligand, galectins, human leukocyte antigen-G5 (HLA-G5) and growth factors such as transforming growth factor-β1 (TGF-β1), hepatic growth factor (HGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor (FGF) and so forth. [2][13][14][15][16][17][18][19]. The use of CM may avoid some problems associated with the therapeutic application of MSCs such as immune rejection of allogeneic cells or undesirable cell differentiation. However, treatment with CM may be an alternative to cellular therapy in regenerative medicine [20].
EVs are main components of MSC secretome which can be incorporated into cells via endocytosis or phagocytosis [21][22][23] leading to the transfer of their content such as proteins, lipids, DNA, RNA, mitochondria and so forth. As a result, EVs regulate gene transcription and the functions of recipient cells [24][25][26][27]. In particular, the transfer of miRNA plays an important role in the biological activity of MSC EVs [28]. Most of the bioactive effects of EVs require the interaction between EV-associated molecules CD9 and CD81 and the binding partners immunoglobulin superfamily, member 8 (IGSF8) and PGF2 receptor negative regulator (PTGFRN) on cells [24]. It is widely accepted that EVs represent an important mechanism for cell communication.
EVs are generally classified into exosomes (EXOs, 30–100 nm in diameter, formed by the inward budding of endosomal membrane to produce a multivesicular body that upon fusion with cell membrane releases these microparticles) and microvesicles (MVs, 50 nm to 1000 nm in diameter, generated by the outward budding and fission of the plasma membrane) (reviewed in Reference [29]). Nevertheless, MSCs may secrete other EVs with overlapping size which do not exhibit the same characteristics as EXOs [30]. As currently there are no appropriate methods to isolate pure EV subtypes, better isolation and characterization methods are under study to establish the necessary protocols and classifications needed to know their specific properties allowing the development of EVs for therapeutic applications. To this respect, the International Society for Extracellular Vesicles (ISEV) is committed to improving the standardization guidelines for EV studies [31].
2. Immune Regulation and Rheumatoid Arthritis
A wide range of evidence indicates that EVs play an essential role in immunomodulation. EVs may either activate or suppress immune responses depending on the type of parent cell [32][33]. However, many in vitro and in vivo studies have demonstrated the predominant anti-inflammatory and immunosuppressive properties of MSC EVs in both innate and adaptive immune cells [34][35]. These therapeutic effects of EVs may be similar to those of parent cells [36]. EVs contain a number of tolerogenic molecules present in MSCs (programmed death-ligand 1, galectin-1 and TGF-β1) [37]. Nevertheless, some studies have reported a higher immunomodulatory ability of MSCs compared with their EVs which may be mediated by cell contact besides a number of factors present in the secretome [38]. Another study has also shown that B cell modulation by MSCs is partially mediated by soluble factors other than EVs [39]. It has been reported that EVs from human bone marrow MSCs (BM-MSCs) reduce the expression of pro-inflammatory cytokines such as IL-1β, IL-6 and tumor necrosis-α (TNFα) in monocytic cells whereas the expression of anti-inflammatory IL-10 and TGF-β1 is enhanced [40]. The anti-inflammatory properties of EVs can be potentiated by stimulation of MSCs with cytokines such as TNFα+interferon γ [41].
MSC EVs exert immunoregulatory effects by several mechanisms (Figure 1). EVs regulate dendritic cell functions with impairment of antigen uptake by immature cells and reductions in cell maturation and activation [42]. Although there are differences depending on the parent cells, MSC EVs may inhibit the proliferation of CD4+ and CD8+ T cells and the promotion of apoptosis in CD4+ T cells [43][44]. EVs may also promote the conversion of T helper type 1 (Th1) into T helper type 2 (Th2) cells whereas differentiation into Th17 cells was decreased [40]. In addition, EVs polarize activated CD4+ T cells to CD4+CD25+FOXP3+ regulatory T cells (Tregs) which requires activation of T cells by antigen presenting cells [43][45][46]. As MSC EVs polarize immune cells toward an immunosuppressive phenotype only in the presence of an activated immune system [46], homeostatic immune activity would not be affected by EVs treatment thus avoiding the increased risk of infection or cancer observed with immunosuppressive drugs [47]. The proliferation and differentiation of B cells and the proliferation of natural killer (NK) cells are reduced by MSC EVs [48][49] while the levels of cytotoxic T lymphocyte-associated protein 4 (CTLA-4), a protein receptor which downregulates immune responses, are enhanced [40]. In particular, EVs have the potential to switch macrophages into an anti-inflammatory phenotype (M2) [50]. Cell pre-conditioning can improve the macrophage differentiation ability of EVs as shown by using hypoxia which increases the content of miRNAs such as miR-223 and miR-146b in EVs [51]. Similarly, treatment of human umbilical cord MSCs (UC-MSCs) with lipopolysaccharide (LPS) has been shown to improve the anti-inflammatory ability of their EXOs through the induction of let-7b which is transferred to recipient cells and targets the toll-like receptor 4 (TLR4)/nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)/signal transducer and activator of transcription 3 (STAT3)/Akt pathway. As a result, macrophages can be polarized into an anti-inflammatory M2 phenotype [52].
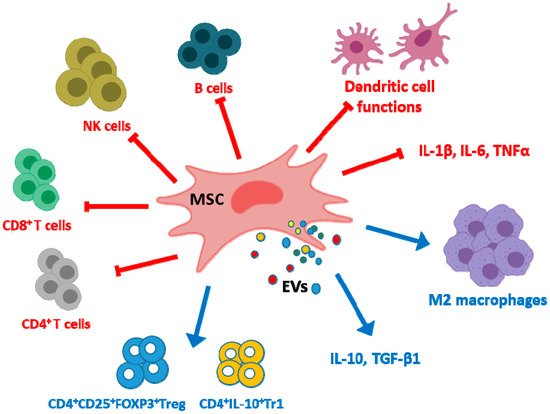
Figure 1. Examples of immunomodulatory effects exerted by mesenchymal stem/stromal cell extracellular vesicles (MSC EVs). EVs inhibit the production of pro-inflammatory cytokines but increase the levels of anti-inflammatory cytokines in monocytic cells. They exert negative effects on dendritic cell maturation and activation with impairment of antigen uptake and also on the proliferation of CD4+ T cells, CD8+ T cells, NK cells and B cells. These EVs promote the conversion of Th1 into Th2 and reduce Th17 differentiation. In contrast, MSC EVs enhance the differentiation of regulatory cells such as M2 macrophages, CD4+CD25+FOXP3+Treg and CD4+IL-10+Tr1.
RA is a chronic autoimmune inflammatory disorder characterized by hyperplasia of the synovial membrane and infiltration of immune and inflammatory cells. Synovial cell transformation, excessive production of inflammatory and catabolic mediators and osteoclast generation progressively result in joint destruction and disability [53][54]. Although there is limited information, EVs may represent a novel therapeutic strategy for arthritis. Experimental evidence indicates an immunomodulatory role for MSC EVs which may counteract antigen-driven T cell responses thus suggesting a potential interest of this approach for the treatment of T cell-mediated diseases such as RA. In a model of antigen (bovine serum albumin)-induced synovitis in pigs, intra-articular injection of EVs from pig BM-MSCs into the carpal joint (500 μg protein/injection in 500 μL) significantly reduced lymphocyte counts and gene expression of TNFα in synovial fluid. In addition, gait analysis at day 7 revealed a trend for the improvement of the impulse which may be due to pain reduction linked to the anti-inflammatory effect of EVs [55].
MVs and EXOs from BM-MSCs isolated from C57BL/6 mice did not reduce the proliferation of CD8+ or CD4+ T lymphocytes but increased CD4+CD25+FOXP3+ Tregs and the CD4+IL10+ Tr1 regulatory cell population. In the in vivo models of delayed-type hypersensitivity and collagen-induced arthritis (CIA) in mice both EV fractions exerted anti-inflammatory effects and protected joints from degradation, with a higher efficacy of EXOs, which may be related to the inhibition of plasmablast differentiation and the induction of IL-10-expressing regulatory B cells [56].
Annexin A1 is an endogenous inhibitory mediator of arthritis [57] enriched in synovial fluid neutrophil EVs from RA patients [58]. Animal models of RA have shown the role of annexin A1 in the anti-inflammatory and chondroprotective effects of neutrophil-derived EVs which depend on the interaction with its receptor formyl peptide receptor 2 (FPR2), inducing anabolic responses with TGF-β1 production and deposition of extracellular matrix, as well as chondrocyte protection from apoptosis [58]. Interestingly, annexin A1 is a main component of adipose tissue-derived mesenchymal stem cells (AD-MSCs) MVs and may contribute to the anti-inflammatory properties of these EVs [59].
3. Bone Repair and Bone Diseases
The secretome of MSCs mediates the mitogenic, pro-migratory and pro-osteogenic effects of these cells promoting bone healing in animal models through the recruitment of MSCs, endothelial cells and progenitor cells [24][60]. It has been reported that EXOs from AD-MSCs enhance the proliferation, migration and differentiation of human osteoblastic cells. These functions are potentiated by TNFα pre-conditioning of AD-MSCs which increases the expression of Wnt-3a in EXOs [61]. BM-MSC EVs promote osteoblast differentiation and expression of osteogenic genes, which is partly mediated by miR-196a as it has been demonstrated in functional experiments [62]. The differentiation stage of progenitor MSCs may influence the miRNA composition of EVs and their ability to induce osteogenic differentiation and mineralization in recipient MSCs [63].
Tissue regeneration requires the recruitment of endogenous stem/progenitor cells to the target site to form tissue specific cells as well as new vessels to provide the necessary oxygen, nutrients and growth factors to repair the affected area [64][65]. The repair effects of EVs on bone have been demonstrated both in vitro and in vivo, in different models of fracture, bone defect or osteoporosis. The relevance of MSC EXOs in bone healing has been shown in a femur fracture model of CD9-/- mice which have impaired EV formation. The retardation of callus formation and fracture healing can be rescued by the injection of BM-MSC EXOs but not by EXOs-free CM [66]. EV administration accelerates bone repair in models of calvarial bone defect in mice and rats. Thus, EVs enhanced osteoblastic proliferation at bone defect edge and stromal progenitor cell migration into the defect mid-substance leading to and increased re-ossification of the defect with increases in bone volume, bone formation area and healing score [24][62]. In addition, EVs from AD-MSCs combined with poly(lactic-co-glycolic acid) (PLGA) scaffolds have demonstrated an enhancing effect on osteogenic, proliferation and migration capabilities of human BM-MSCs in vitro. This approach also enhanced bone regeneration through these osteoinductive effects in critical-sized calvarial defect in mice [67]. Human induced pluripotent stem cells (iPSCs) obtained via genetic reprogramming of adult somatic cells may have some advantages compared with adult MSCs for therapeutic applications. Autologous iPSCs-derived MSCs (iMSCs) have great potential in regenerative medicine and they are superior to MSCs in cell proliferation ability, immunomodulation and secretion of bioactive factors including EVs [68][69]. Therefore, EXOs derived from human iMSCs combined with tricalcium phosphate (β-TCP) improved the osteoinductivity of these scaffolds through activation of phosphatidylinositol-3-kinase (PI3K)/Akt signaling in human BM-MSCs [70].
In bone tissue repair, MSC EVs promote osteogenesis and angiogenesis (Figure 2) which may depend on interactions with different cell types such as BM-MSCs and endothelial cells. EVs from MSCs can be useful tools in bone regenerative medicine as they induce lineage specific differentiation of BM-MSCs and can bind to extracellular matrix proteins such as type I collagen and fibronectin [71]. Nevertheless, the source of EVs influences these regenerative properties. Thus, EXOs from diabetic rat BM-MSCs showed a reduced effect on the osteogenic differentiation of BM-MSCs and the angiogenic activity of human umbilical cord endothelial cells, compared with EXOs from healthy rats. In addition, bone regeneration and neovascularization effects in rat calvarial defects were lower for EXOs from diabetic rats [72].
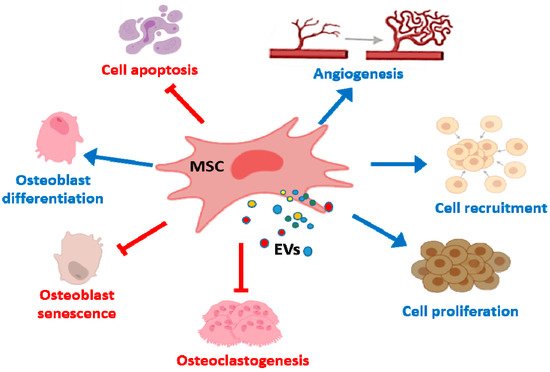
Figure 2. Effects of MSC EVs on bone repair and bone diseases. MSC EVs enhance proliferation, migration and osteogenic differentiation of BM-MSCs whereas apoptosis is inhibited. In addition, proliferation and recruitment of endothelial cells/endothelial progenitor are promoted leading to angiogenesis. MSC EVs also reduce osteoclast formation and osteocyte-like cells apoptosis.
EVs may be provide novel opportunities in endochondral repair of large bone defects which need bone grafts and costly interventions (reviewed in Reference [73]). Thus, EVs may be a better approach compared with cell-based therapies as implanted cells compete with endogenous progenitor cells for oxygen and nutrients in this ischemic microenvironment. In particular, MSCs cannot adapt their glucose consumption and do not possess the necessary glycolytic reserves to maintain their metabolism [74]. In fact, reduction of MSCs metabolic needs by induction of quiescence can enhance their survival under ischemic conditions in the lesion site [75] while hypoxia can reduce their osteogenic potential [76]. In addition, most culture-expanded MSCs cannot adapt to the microenvironment and they die or are phagocytosed by macrophages shortly after implantation in critical-sized bone defects. Distraction osteogenesis induces bone tissue regeneration in large bone defects through the recruitment of endogenous MSCs and endothelial cells/endothelial progenitor cells to the distraction gap. Administration of BM-MSC CM is an effective procedure to accelerate this process as it promotes the migration of Sca-1+/PDGFR-α+ BM-MSCs which differentiate into osteoblasts and CD31+ cells leading to angiogenesis in a mouse model. Soluble factors CCL-2, CCL-5 and CCL-7 can recruit bone marrow mononuclear cells while IL-3 and IL-6 recruit endothelial cells and progenitor cells to enhance osteogenic activity [60]. Administration of EVs from different origins may be another strategy to shorten these lengthy procedures. Recently, it has been demonstrated a role for EXOs from progenitor endothelial cells in the stimulation of angiogenesis thus accelerating bone regeneration during distraction osteogenesis [77].
Besides EVs from BM-MSCs and AD-MSCs, EVs from different MSCs are of interest in bone repair. Therefore, EXOs derived from UC-MSCs enhance fracture healing by promoting angiogenesis in a rat model of stabilized fracture. These EXOs are taken up by endothelial cells leading to hypoxia-inducible factor-1α (HIF-1α) activation and VEGF production with the result of cell proliferation, migration and tube formation [78].
Experimental evidence also indicates the potential application of MSC EVs in osteoporosis. To this respect, implantation of EXOs secreted by iMSCs incorporated in β-TCP scaffolds into critical size calvarial bone defects enhanced osteogenesis and angiogenesis in ovariectomized rats, a model of postmenopausal osteoporosis. In vitro experiments demonstrated the ability of these EVs to stimulate the proliferation and osteogenic differentiation of BM-MSCs from ovariectomized rats [79]. Autologous EVs from human urine-derived stem cells are a potential therapeutic agent for osteoporosis. Systemic injection of these EVs have been shown to promote osteogenesis and inhibit osteoclastogenesis in osteoporotic mice by transferring collagen triple-helix repeat containing 1 (CTHRC1) and osteoprotegerin proteins [80].
Senile osteoporosis may be another potential application of EVs. Therefore, bone loss was attenuated by i.v. administration of EVs from human umbilical cord blood plasma to 16 months old male C57BL/6 mice. This treatment increased trabecular and cortical bone mass and promoted osteoblast differentiation while reducing osteoclast formation. miR-3960 was enriched in these EVs and mediates their osteogenic effects [81]. Increased osteocyte apoptosis and osteoclast activation leads to imbalanced bone remodeling during ageing. AD-MSC EVs reduced hypoxia/serum deprivation-induced apoptosis of osteocyte-like cells through upregulation of Bcl-2/Bax and downregulation of reactive oxygen species (ROS), cytochrome c and activation of caspase-9 and caspase-3. In addition, these EVs reduced osteoclastogenesis suggesting the potential of AD-MSC EVs to improve age-related bone loss [82].
Apoptosis of bone marrow cells plays an important role in the destruction of bone tissue during long-term or high dose glucocorticoid treatment leading to osteonecrosis of the femoral head. Strategies able to prevent apoptosis and promote proliferation of bone marrow cells may be useful to start tissue repair and inhibit disease progression. One of these strategies is the application of MSCs or EVs. Therefore, EXOs derived from human synovial MSCs are internalized by bone marrow cells to promote cell proliferation and inhibition of apoptosis. In a rat model of osteonecrosis of the femoral head induced by methylprednisolone, i.v. administration of 1 × 1011 particles of EXOs (in 200 μL) exerted a preventive effect and counteracted the inhibition of the osteogenic response induced by glucocorticoid [83]. It is known that EXOs secreted by human iMSCs promote angiogenesis due to the activation of PI3K/Akt signaling in endothelial cells. As a consequence, i.v. administration in the steroid-induced rat osteonecrosis model prevented bone loss and osteonecrosis of the femoral head [84]. As HIF-1α plays a role in osteogenesis and angiogenesis [85][86], strategies directed at the modulation of this transcription factor may improve the bone repair properties of EVs. Therefore, BM-MSCs transfected with a mutant HIF-1α which is not degraded in normoxic conditions were used to obtain mutant EVs which exhibited enhanced osteogenesis and angiogenesis properties leading to the repair of steroid-induced avascular necrosis of the femoral head in rabbits [87].
Potential therapeutic applications of MSCs from a variety of sources have been extended to periodontal defects [88]. Periodontitis leads to pathologic loss of periodontal ligament, cementum and alveolar bone [89]. Receptor activator of NF-κB ligand (RANKL) plays a main role in osteoclastogenesis during inflammatory bone resorption and activated T and B cells can be the source of this cytokine in periodontal disease [90]. MSCs from different origins have shown a periodontal regenerative potential in animal models without adverse effects. In particular, local implantation of periodontal ligament-derived MSCs have demonstrated their ability in the regeneration of periodontal ligament and cementum [88]. Similarly, collagen sponges containing CM from BM-MSCs have been shown to repair periodontal defects in rats 4 weeks after implantation with regeneration of alveolar bone, cementum and periodontal ligament. This CM contains a number of growth factors such as insulin-like growth factor-1, VEGF, TGF-β1 and HGF which could mediate mobilization of endogenous MSCs, angiogenesis and differentiation [91]. Interestingly, CM from BM-MSC stimulates alveolar bone augmentation prior to dental implant placement in humans without inducing an inflammatory response [92]. Implants of collagen sponges including EXOs (from HuES9.E1 cells-derived MSCs) have been shown to increase the formation of new alveolar bone in a rat model of surgically created periodontal intrabony defects. These EXOs could increase rat periodontal ligament cell migration and proliferation through CD73-mediated adenosine receptor activation of pro-survival Akt and ERK signaling pathways [93].
References
- World Health Organization. Musculoskeletal Conditions. Available online: https://www.who. int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 21 November 2019).
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131.
- Wang, L.; Wang, L.; Cong, X.; Liu, G.; Zhou, J.; Bai, B.; Li, Y.; Bai, W.; Li, M.; Ji, H.; et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem Cells Dev. 2013, 22, 3192–3202.
- Franceschetti, T.; De, B.C. The potential role of adult stem cells in the management of the rheumatic diseases. Ther. Adv. Musculoskelet. Dis. 2017, 9, 165–179.
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. BMC Musculoskelet. Disord. 2016, 17, 230.
- Vega, A.; Martin-Ferrero, M.A.; Del, C.F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690.
- Cui, G.H.; Wang, Y.Y.; Li, C.J.; Shi, C.H.; Wang, W.S. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: A meta-analysis. Exp. Ther. Med. 2016, 12, 3390–3400.
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult stem cells for bone regeneration and repair. Front. Cell Dev. Biol. 2019, 7, 268.
- Marolt, P.D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal stromal cell-based bone regeneration therapies: From cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352.
- Jo, C.H.; Yoon, K.S.; Chai, J.W.; Jeong, E.C.; Oh, S. Intratendinous injection of mesenchymal stem cells for the treatment of rotator cuff disease: A 2-year follow-up study. Arthroscopy 2019.
- Klimczak, A.; Kozlowska, U.; Kurpisz, M. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch. Immunol. Ther. Exp. 2018, 66, 341–354.
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837.
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621.
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822.
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150.
- Chabannes, D.; Hill, M.; Merieau, E.; Rossignol, J.; Brion, R.; Soulillou, J.P.; Anegon, I.; Cuturi, M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007, 110, 3691–3694.
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402.
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222.
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843.
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586.
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312.
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell Biochem. 2010, 111, 488–496.
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687.
- Xu, J.; Wang, Y.; Hsu, C.Y.; Gao, Y.; Meyers, C.A.; Chang, L.; Zhang, L.; Broderick, K.; Ding, C.; Peault, B.; et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. eLife 2019, 8, e48191.
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286.
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles and friends. J. Cell Biol. 2013, 200, 373–383.
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 320.
- Van, N.G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Lai, R.C. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208.
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem cell-derived extracellular vesicles and immune-modulation. Front. Cell Dev. Biol. 2016, 4, 83.
- Siegel, G.; Schafer, R.; Dazzi, F. The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009, 87, S45–S49.
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Front. Immunol. 2019, 10, 2663.
- Fierabracci, A.; Del, F.A.; Luciano, R.; Muraca, M.; Teti, A.; Muraca, M. Recent advances in mesenchymal stem cell immunomodulation: The role of microvesicles. Cell Transplant. 2015, 24, 133–149.
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54.
- Conforti, A.; Scarsella, M.; Starc, N.; Giorda, E.; Biagini, S.; Proia, A.; Carsetti, R.; Locatelli, F.; Bernardo, M.E. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014, 23, 2591–2599.
- Carreras-Planella, L.; Monguio-Tortajada, M.; Borras, F.E.; Franquesa, M. Immunomodulatory effect of MSC on B cells is independent of secreted extracellular vesicles. Front. Immunol. 2019, 10, 1288.
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840.
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells 2018, 36, 79–90.
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X.N. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front. Immunol. 2018, 9, 2538.
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442.
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front. Immunol. 2014, 5, 556.
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244.
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy 2018, 20, 687–696.
- Toh, W.S.; Zhang, B.; Lai, R.C.; Lim, S.K. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy 2018, 20, 1419–1426.
- Budoni, M.; Fierabracci, A.; Luciano, R.; Petrini, S.; Di Ciommo, V.; Muraca, M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013, 22, 369–379.
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Kamga, P.T.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016, 6, 24120.
- Henao Agudelo, J.S.; Braga, T.T.; Amano, M.T.; Cenedeze, M.A.; Cavinato, R.A.; Peixoto-Santos, A.R.; Muscará, M.N.; Teixeira, S.A.; Cruz, M.C.; Castoldi, A.; et al. Mesenchymal stromal cell-derived microvesicles regulate an internal pro-inflammatory program in activated macrophages. Front. Immunol. 2017, 8, 881.
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028.
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308.
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361.
- Goronzy, J.J.; Weyand, C.M. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, 249.
- Casado, J.G.; Blazquez, R.; Vela, F.J.; Alvarez, V.; Tarazona, R.; Sanchez-Margallo, F.M. Mesenchymal stem cell-derived exosomes: Immunomodulatory evaluation in an antigen-induced synovitis porcine model. Front. Vet. Sci. 2017, 4, 39.
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noel, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410.
- Yang, Y.; Hutchinson, P.; Morand, E.F. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 1999, 42, 1538–1544.
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’Accio, F.; Pitzalis, C.; et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015, 7, 315ra190.
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25.
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90.
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.; Zreiqat, H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng. Part A 2017, 23, 1212–1220.
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961.
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekstrom, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059.
- Shirley, D.; Marsh, D.; Jordan, G.; McQuaid, S.; Li, G. Systemic recruitment of osteoblastic cells in fracture healing. J. Orthop. Res. 2005, 23, 1013–1021.
- Lee, D.Y.; Cho, T.J.; Kim, J.A.; Lee, H.R.; Yoo, W.J.; Chung, C.Y.; Choi, I.H. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 2008, 42, 932–941.
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016, 5, 1620–1630.
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces. 2018, 10, 5240–5254.
- Hirschi, K.K.; Li, S.; Roy, K. Induced pluripotent stem cells for regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 277–294.
- Sabapathy, V.; Kumar, S. hiPSC-derived IMSCs: Nextgen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell Mol. Med. 2016, 20, 1571–1588.
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136.
- Narayanan, R.; Huang, C.C.; Ravindran, S. Hijacking the cellular mail: Exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016, 2016, 3808674.
- Zhu, Y.; Jia, Y.; Wang, Y.; Xu, J.; Chai, Y. Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl. Med. 2019, 8, 593–605.
- Ferreira, E.; Porter, R.M. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res. 2018, 7, 263–273.
- Moya, A.; Paquet, J.; Deschepper, M.; Larochette, N.; Oudina, K.; Denoeud, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells 2018, 36, 363–376.
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence preconditioned human multipotent stromal cells adopt a metabolic profile favorable for enhanced survival under ischemia. Stem Cells 2017, 35, 181–196.
- Potier, E.; Ferreira, E.; Andriamanalijaona, R.; Pujol, J.P.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007, 40, 1078–1087.
- Jia, Y.; Zhu, Y.; Qiu, S.; Xu, J.; Chai, Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019, 10, 12.
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570.
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016, 12, 836–849.
- Chen, C.Y.; Rao, S.S.; Tan, Y.J.; Luo, M.J.; Hu, X.K.; Yin, H.; Huang, J.; Hu, Y.; Luo, Z.W.; Liu, Z.Z.; et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019, 7, 18.
- Hu, Y.; Xu, R.; Chen, C.Y.; Rao, S.S.; Xia, K.; Huang, J.; Yin, H.; Wang, Z.X.; Cao, J.; Liu, Z.Z.; et al. Extracellular vesicles from human umbilical cord blood ameliorate bone loss in senile osteoporotic mice. Metabolism 2019, 95, 93–101.
- Ren, L.; Song, Z.J.; Cai, Q.W.; Chen, R.X.; Zou, Y.; Fu, Q.; Ma, Y.Y. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun. 2019, 508, 138–144.
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Sheng, J.G.; Zhang, C.Q. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int. J. Biol. Sci. 2016, 12, 1262–1272.
- Liu, X.; Li, Q.; Niu, X.; Hu, B.; Chen, S.; Song, W.; Ding, J.; Zhang, C.; Wang, Y. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017, 13, 232–244.
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 2007, 117, 1616–1626.
- Kim, H.H.; Lee, S.E.; Chung, W.J.; Choi, Y.; Kwack, K.; Kim, S.W.; Kim, M.S.; Park, H.; Lee, Z.H. Stabilization of hypoxia-inducible factor-1alpha is involved in the hypoxic stimuli-induced expression of vascular endothelial growth factor in osteoblastic cells. Cytokine 2002, 17, 14–27.
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1alpha-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390.
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 2017, 52, 793–812.
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol 2000 2017, 75, 7–23.
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998.
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381.
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face. Med. 2016, 12, 5.
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu, J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
752
Revisions:
2 times
(View History)
Update Date:
14 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




