Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federico Gulluni | + 4462 word(s) | 4462 | 2021-07-23 03:35:44 | | | |
| 2 | Lily Guo | -173 word(s) | 4289 | 2021-09-30 03:17:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gulluni, F. Phosphoinositide 3-kinase and Breast Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/14754 (accessed on 08 February 2026).
Gulluni F. Phosphoinositide 3-kinase and Breast Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/14754. Accessed February 08, 2026.
Gulluni, Federico. "Phosphoinositide 3-kinase and Breast Cancer" Encyclopedia, https://encyclopedia.pub/entry/14754 (accessed February 08, 2026).
Gulluni, F. (2021, September 29). Phosphoinositide 3-kinase and Breast Cancer. In Encyclopedia. https://encyclopedia.pub/entry/14754
Gulluni, Federico. "Phosphoinositide 3-kinase and Breast Cancer." Encyclopedia. Web. 29 September, 2021.
Copy Citation
Breast cancer is the most frequently diagnosed cancer and the primary cause of cancer death in women worldwide. Although early diagnosis and cancer growth inhibition has significantly improved breast cancer survival rate over the years, there is a current need to develop more effective systemic treatments to prevent metastasis. One of the most commonly altered pathways driving breast cancer cell growth, survival, and motility is the PI3K/AKT/mTOR signaling cascade.
Phosphoinositide 3-kinase (PI3K) is a group of lipid kinases that phosphorylate the 3′-OH group of phosphatidylinositol (PI) at plasma and intracellular membranes.
PI3K
inhibitor
AKT
mTOR
breast cancer
1. Introduction
Phosphoinositide 3-kinase (PI3K) is a group of lipid kinases that phosphorylate the 3′-OH group of phosphatidylinositol (PI) at plasma and intracellular membranes. They are split into three different classes according to their structure, binding partners, and substrate specificity [1][2][3]. Among them, the most well-studied PI3K is class I PI3K, which generate PI(3,4,5)P3 (PIP3) starting from PI(4,5)P2 (PIP2). PIP3 is mainly produced at the plasma membrane in response to different stimuli and allows for the recruitment of a myriad of phospholipid effectors, including serine/threonine kinase AKT and 3-phosphoinositide-dependent protein kinase-1 (PDK-1), which are the central mediators of the PI3K pathway. PIP3 also facilitates PDK1 and AKT interaction, resulting in phosphorylation of AKT at thrPhosphorylated AKT promotes protein synthesis, cell growth, and cell survival and motility by activating many downstream kinases, including the mammalian target of the rapamycin (mTOR) complex [1]. On the other hand, the tumor suppressor phosphatase and tensin homolog (PTEN) dephosphorylates PIP3, counteracting PI3K signaling [4] (Figure 1).
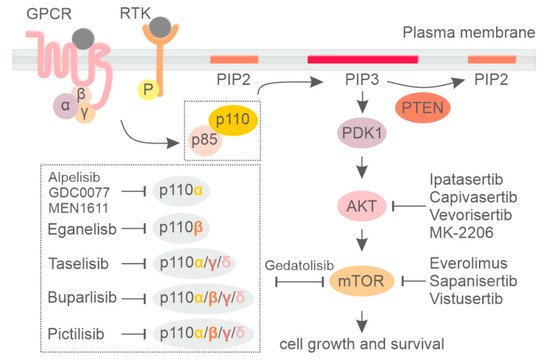
Figure 1. Signaling by the phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of the rapamycin (mTOR) pathway and the respective inhibitors.
Class I PI3K includes four highly homologous catalytic subunits, p110α, p110β, p110γ, and p110δ, which can associate with five regulatory subunits, collectively referred to as p85-type regulatory subunits (Figure 1) [5][6]. Whereas p110α and p110β isoforms are ubiquitously expressed, p110δ and p110γ expression is largely restricted to hematopoietic cells [7][8]. Dysregulation of phosphoinositide kinases, primarily in class IA PI3K, have been discovered in a number of human diseases, with mutations leading to either increased or decreased enzymatic activity being critically involved in cancer [9], developmental disorders [10], and primary immune deficiencies [11][12][13]. For an extensive description of the PI3K pathway, the reader can refer to the following reviews [1][14][15].
Several studies suggest that the PI3K/AKT/mTOR pathway is often genetically altered in human cancers [15][16]. Although many small molecule inhibitors targeting the PI3K/AKT/mTOR signaling pathway were pre-clinically studied, only some of the PI3K and mTOR inhibitors are currently approved for the treatment of human cancers in the clinic. Here we summarize the most recent advances in the inhibition of the PI3K/AKT/mTOR signaling pathway in breast cancer.
2. PI3K Isoform-Specific Inhibitors
Considering the limitations encountered with pan-PI3K inhibitors, the selective inhibition of specific PI3K isoforms permits the administration of therapeutic doses of drugs, avoiding severe off-target toxicity. On the other hand, a selective approach demands a precise strategy to select patients who may benefit from that treatment [17]. In breast cancer, activating mutations in the PIK3CA gene are the most frequent alteration of the PI3K pathway, leading to hyperactivation of p110α. Therefore, great efforts have been directed to developing PIK3CA-selective inhibitors to specifically target this PI3K isoform.
-
Alpelisib
Alpelisib (BYL719) is the first oral PI3K inhibitor selectively targeting the p110α isoform (Table S1). Its efficacy was first assessed in preclinical models showing potent inhibition over the two most common PIK3CA mutations (H1047R and E545K) at nanomolar concentration (4.6 nM/L) [18]. Notably, treatment with alpelisib not only interfered with PIK3CA-mediated downstream signaling, but also induced a dose-dependent decrease in p110α protein levels in ER+/PIK3CA-mutated breast cancer cell lines [19], suggesting a dual mode of action. The combination of alpelisib with fulvestrant also demonstrated synergism between the two drugs in xenograft models [20]. A tolerable safety profile and encouraging activity in patients with PIK3CA-altered solid tumors was reported for alpelisib in a first-in-human phase I study [21]. The subsequent phase II clinical trial was conducted to assess the maximum tolerable dose, safety, and efficacy of alpelisib in combination with fulvestrant in HR+/HER2– metastatic breast cancer [22]. Partial or complete response was observed among 29% of pretreated metastatic breast cancer patients with PIK3CA alterations, whereas no tumor response was reported in the PIK3CA wild-type group [22]. A favorable safety profile in these patients included mainly on-target effects such as hyperglycemia, nausea, and diarrhea [22].
Next, a phase III SOLAR-1 clinical trial evaluated the efficacy and safety of alpelisib in combination with hormonal therapy (fulvestrant) in HR+/HER2– metastatic breast cancer patients who recurred or progressed after endocrine therapy [23]. Patients were selected and stratified based on the PIK3CA mutational status to also include a cohort of PIK3CA wild type as proof of activity in this subgroup. PIK3CA status was determined by tumor tissue RT-PCR and led to the inclusion of 341 patients in the PIK3CA-mutant cohort and 231 in the wild-type group. The primary and secondary endpoints for this study were to evaluate the PFS and the overall survival in patients with PIK3CA genetic alterations together with the safety and efficacy in the PIK3CA wild-type group. The median PFS of patients with PIK3CA genetic alterations was 11.0 months in the alpelisib/fulvestrant arm versus 5.7 months in the placebo/fulvestrant arm (HR 0.65 95% CI 0.50–0.85; p < 0.001). The overall response was also higher with alpelisib/fulvestrant compared to placebo/fulvestrant (26.6% and 12.8%, respectively). Conversely, in the PIK3CA wild-type group, alpelisib administration was not significantly associated with improved PFS (7.4 versus 5.6 months, respectively; HR 0.85; 95% CI, 0.58–1.25). Toxicity due to alpelisib administration was associated with specific p110α inhibition and included hyperglycemia (63.7% versus 9.8% for the alpelisib and placebo arms, respectively), diarrhea (57.7% versus 15.7%, respectively), and rash (35.6% versus 5.9%, respectively). Permanent discontinuation due to AEs occurred in 25% of patients in the alpelisib group versus 4.2% in the placebo arm. The positive results from the SOLAR-1 trial prompted the Food and Drug Administration (FDA) to approve the combination of alpelisib with fulvestrant for the treatment of men and postmenopausal women with HR+/HER2–, PIK3CA-mutated, advanced, or metastatic breast cancer, as detected by an FDA-approved test following progression on or after an endocrine-based regimen. One year after the approval of alpelsib, the data of overall survival were released. Although overall survival with a median follow-up of 30.8 months did not meet statistical significance, the absolute difference of 8 months observed between the alpelisib versus the placebo treatment was clinically relevant and valuable, with PFS benefit not only maintained but also increased in terms of overall survival outcome [24].
The SOLAR-1 clinical trial started to recruit the first patients during the second half of 2015, a few months after the FDA granted accelerated approval for the CDK4/6 inhibitor palbociclib in combination with endocrine treatment for postmenopausal HR+/HER2- metastatic breast cancer [25][26]. For this reason, only 5% of patients with mutations in PIK3CA included in SOLAR-1 had received a CDK4/6 inhibitor before being enrolled in the clinical trial. To better evaluate the efficacy of alpelisib in patients treated with a CDK4/6 inhibitor, the phase II BYLieve trial was designed [27]. In this trial, patients were enrolled based on their previous treatment with an aromatase inhibitor in combination with CDK4/6 and received fulvestrant plus alpelisib. Almost 50% of patients showed no disease progression at 6 months. Median PFS also resulted in 7.3 months [27], in line with previous results from the SOLAR-1 subgroup analysis in which 44% of patients (9 out of 20) receiving fulvestrant plus alpelisib were alive without disease progression at 6 months and a median PFS of 5.5 months [23]. These findings support the use of alpelisib in combination with fulvestrant after CDK4/6 inhibitors [23][27].
-
Taselisib
Taselisib (GDC-0032, Genentech, San Francisco, CA) is an oral PI3K inhibitor equally inhibiting p110α, δ, and γ isoforms of class I PI3K but with 30-fold less potency against p110β [28] (Table S1). Given its greater selectivity against PI3K isoforms, taselisib was expected to have improved efficacy on PIK3CA-mutant tumors and less toxic effects compared to pan-PI3K inhibitors. In particular, treatment with taselisib resulted in marked tumor suppression in preclinical studies performed on PIK3CA-mutant xenografts [29]. An initial phase I clinical trial demonstrated clinical activity of taselisib in patients with advanced solid tumors, particularly in breast cancers with PIK3CA genetic alterations, with an overall response of 36% compared to no response in patients with wild-type PIK3CA [30]. Based on encouraging phase I results, a phase III clinical trial called SANDPIPER was performed on postmenopausal ER+/PIK3CA-mutated metastatic breast cancer patients previously treated with AI. Fulvestrant was administered in combination with either taselisib or placebo and the primary endpoint was the assessment of PFS in patients with PIK3CA-mutated tumors (>80% of participants) [31]. Median PFS was significantly longer, although modest, in the taselisib arm (7.4 months) versus the placebo arm (5.4 months) (HR 0.7 95% CI 0.56–0.89; p = 0.004). Patients treated with taselisib also had a significantly higher objective response rate compared to placebo (28% and 11.9%, respectively). Treatment with taselisib was also associated with higher severe adverse effects, including diarrhea (grade 3/4 of 12% for taselisib arm versus <1% for placebo) and hyperglycemia ((grade 3/4 11% versus <1%, respectively). Because the clinical benefits observed in the SANDPIPER trial were modest and the tolerability was questionable, further investigation of taselisib was stopped [31]. One of the reasons accounting for the lack of efficacy of taselisib is likely related to the less potent and specific inhibition of p110a compared to alpelisib, as evidenced by the higher rates of hyperglycemia in the SOLAR-1 trial compared to the SANDPIPER trial [18][32].
3. PI3K Pathway Inhibition in HER2+ and Triple-Negative Breast Cancer Subtypes
-
HER2-Positive Breast Cancer
Preclinical studies demonstrated that HER2 signaling largely relies on p110α rather than on other class-I PI3K isoforms [33], thus providing a strong rationale for therapeutic intervention and targeting of PIK3CA in HER2+ breast tumors. Particularly, reduced pathological complete response (pCR) rate was linked to PIK3CA mutational status in HER+ breast cancer patients who received neoadjuvant chemotherapy and anti-HER2 therapy [34]. Some clinical trials were conducted to determine the potential benefit of inhibiting PI3K in HER2+ breast tumors. A phase I study called PIKHER2 was designed to assess the effect of combining pan-PI3K inhibitors buparlisib and lapatinib in trastuzumab-resistant HER2+ metastatic breast cancer independently of PIK3CA mutational status [35]. The observed clinical benefit rate (CBR) was 29%, and complete response was observed in one patient (4%). Another phase Ib/II clinical trial tested the combination of buparlisib with trastuzumab in HER2+ breast tumors resistant to trastuzumab [36]. Also in this case, the trial was conducted without considering the PIK3CA mutational status. Although the authors evidenced some clinical activity with the combination (2% complete response and 8% partial response), the trial failed to reach the estimated primary endpoint of objective response rate >25%.
The NeoPHOEBE phase II clinical trial enrolled HER2+ early breast tumors to be treated with either buparlisib or placebo in combination with paclitaxel and trastuzumab [37]. In this setting, the percentage of patients with genetic alteration in PIK3CA was below 20%. The authors observed a pCR rate of 32% for the buparlisib group compared to 40% in the placebo group. In line with other trials conducted on buparlisib, its administration was associated with higher toxicity, leading to 36% of adverse events compared to less than 10% in the placebo arm.
Besides pan-PI3K inhibitors, a phase I trial was conducted with the p110α-specific alpelisib inhibitor in association with trastuzumab emtansine (TDM-1) in trastuzumab-resistant breast cancer patients [38]. The objective response rate of this study was 43%, with 60% of clinical benefit rate specifically in patients who had previously progressed on TDM-Moreover, 53% of patients included in the study presented alteration of the PI3K pathway, including PIK3CA mutations, PTEN loss, or AKT overexpression. Almost half of these patients showed clinical benefit rate, even in case of previous progression in TDM-1 therapy [38]. Adverse effects (grade > 3) occurred in 59% of patients but they were generally manageable. These findings demonstrated that activation of the downstream PI3K pathway can be a possible mechanism of tumor resistance to TDM-1 [39].
Other alpha-specific class I PI3K inhibitors are currently being tested in clinical trials to target the PI3K pathway in breast cancer patients. Among them, GDC-0077 is a new potent, orally available, and p110α-selective inhibitor. It has already shown robust activity in preclinical models of breast tumors with genetic alterations in PIK3CA [40][41]. Mechanistically, GDC-0077 leads to downregulation of p110α, thus interfering with the activation of PI3K downstream targets such as the phosphorylation of AKT. Accordingly, treatment of human PIK3CA-mutant breast cancer cell lines with GDC-0077 resulted in reduced proliferation and increased apoptosis. Similar results were observed in xenograft models where GDC-0077 was combined with standard-of-care treatments for HR-positive breast cancer such as anti-estrogen (fulvestrant) or CDK4/6 inhibitor (palbociclib) [41]. An ongoing phase I trial showed that GDC-0077 in association with palbociblib and fulvestrant can be combined at maximum doses. INAVO120 is a phase III, randomized, double-blind, pbo-controlled study that will assess the efficacy and safety of GDC-0077/pbo plus palbociblib and fulvestrant in patients with PIK3CA-mutant/HR+/HER2– advanced metastatic breast cancer [42].
Another novel PI3K inhibitor, targeting the mutated form of p110α and p110γ, is MEN1611 [43]. In both xenografts and PDX models of breast cancer, MEN1611 showed significant activity either as a monotherapy or in combination with targeted therapies in breast cancer and other solid tumors. In HER2+ breast cancer cell lines mutated for PIK3CA, as well as in patient-derived xenograft models, MEN1611 seemed to act synergistically when associated with trastuzumab, also inducing a dose-dependent p110α protein depletion and a pro-inflammatory phenotype compatible with p110γ inhibition [44].
-
Triple-Negative Breast Cancer (TNBC)
In the other types of breast cancer, there is a shortage of clinical trials of alpelisib application. For example, in triple-negative breast cancer (TNBC), a phase I clinical trial is testing the effect of chemotherapy combining alpelisib with enzalutamide in AR+ and PTEN+ breast cancer, including a cohort of TNBC (NCT03207529). Another phase III study is assessing the efficacy and safety of alpelisib plus nab-paclitaxel in subjects with advanced TNBC with PIK3CA mutation. The results from these ongoing trials will provide us a better perspective on how alpelisib affects triple-negative breast cancer patients. Similar results have been reported in a phase II neoadjuvant-based clinical trial (NCT02273973).
A phase I clinical trial (NCT01884285) is also studying the PI3KCB/PI3KCD inhibitor AZD8186 in patients with TNBC and known PTEN-deficient/-mutated or PIK3CB-mutated/-amplified advanced tumors and in combination with abiraterone acetate or AZD2014, an mTOR inhibitor [45]. AZD8186 has single-agent efficacy in PTEN-deficient TNBC cell lines in vitro, but has limited single-agent efficacy in vivo [46]. However, AZD8186 showed enhanced efficacy when combined with paclitaxel and anti-PD1 in vivo [46]. Further study is needed to determine the optimal combination therapies for PTEN-deficient breast cancer.
Immuno-oncology is also gaining increasing interest as a valuable therapeutic strategy in breast cancer [15][47]. TNBC is considered the most immunogenic subtype of breast cancer, with a higher lymphocyte infiltration rate than HER2+ or HR+ tumors and thus is regarded as a promising target for immunotherapies [48]. MARIO-3 (NCT03961698) is a phase 2 clinical study designed to evaluate IPI-549 (eganelisib), Infinity Pharmaceutical’s oral immuno-oncology product targeting immuno-suppressive tumor-associated myeloid cells through selective inhibition of PI3KCG, in combination with Tecentriq (atezolizumab) and Abraxane (nab-paclitaxel) in front-line TNBC. The novel triplet regimen of IPI-549, atezolizumab, and nab-paclitaxel showed promising antitumor activity irrespective of biomarker status, with manageable toxicity. The expansion phase of the phase II study is currently enrolling, with a target completion date of 2022 [49].
4. Currently Available Inhibitors Acting on AKT and mTOR in Breast Cancer
-
AKT Inhibitors
AKT consists of three isoforms (AKT1, AKT2, and AKT3). It is the major downstream target of PI3K and one of the most common molecular alterations in cancer [50]. Targeting of this altered pathway by pharmacologic modulation of AKT activity represents a powerful strategy for cancer intervention [51]. Among different AKT inhibitors (Table S1), AZD5363 (capivasertib) has been used as a monotherapy in breast cancer in a phase I, open-lab study for patients with AKT E17K mutations [52]. Capivasertib was well tolerated and achieved plasma levels and robust modulation of AKT activity in tumors. Proof-of-concept responses were observed in patients with PIK3CA-mutant cancers treated with AZD5363 [52]. Another pan-AKT inhibitor, GDC-0068 (ipatasertib) has been used as a monotherapy in triple-negative breast cancer cases and has already entered phase I and II studies [53]. Dose-limiting side effects during treatment together with dose reduction occurred in both trials and were mainly due to the fact that the ATP-binding pocket of AKT is highly conserved among other kinases, which limits selectivity [54].
Major efforts are now directed towards the identification of AKT-specific and isoform-selective small molecules. For instance, MK-2206 and miransertib (ARQ092) are bioactive allosteric inhibitors that offer greater specificity, reduced side effects, and lower toxicity compared to other targeted approaches [50][55]. In a clinical trial (I-SPY TRIAL, NCT01277757), MK-2206 is currently tested in combination with or without trastuzumab for treatment of advanced breast cancer with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation [56]. However, MK-2206 monotherapy had limited clinical activity in advanced breast cancer patients due to tumor heterogeneity and tolerable dose. Similarly, MK-2206 is unlikely to add further benefit to the efficacy of anastrozole alone in a phase II study based on PIK3CA-mutant ER+ breast cancers (NCT01776008). Future study designs should consider emerging data regarding population subtypes that may benefit most from specific drug combinations.
Another phase 1b study of the miransertib next-generation inhibitor ARQ 751 (vevorisertib, NCT02761694) as a single agent or in combination with either paclitaxel or fulvestrant in patients with advanced solid tumors with PIK3CA/AKT/PTEN mutations was recently completed, although results are not yet available. The pan-inhibitor MK2206 remains the most prominent of the allosteric inhibitors; however, others such as TAS-117 have also shown promising effects [50][57][58].
Another innovative approach to targeting AKT in disease involves the irreversible covalent modification of two noncatalytic cysteines in the activation loop of AKT by covalent–allosteric inhibitors (CAAIs), such as borussertib. The in vivo efficacy of borussertib was proven in combination studies with MEK-inhibitor trametinib in KRAS-mutant patient-derived xenograft models, leading to a partial response [55][59]. Further studies are required to better understand its clinical relevance, particularly in breast cancer.
-
mTOR Inhibitors
mTOR is one of the most important downstream effectors of the PI3K/AKT pathway. Inhibitors targeting mTOR, including everolimus (RAD001), MLN0128, and AZD014, have been broadly studied and evaluated in hematological cancer and solid tumors [60] (Table S1). Everolimus and its combination with exemestane has been approved by the FDA for the treatment of hormone receptor-positive/HER2-negative (HR+/HER2−) breast cancer [61][62]. This synergistic effect was also observed in postmenopausal women with metastatic ER+/HER− breast tumor. In this study, a combination of everolimus and tamoxifen showed a significant reduction in cancer progression and increased overall survival rate compared to tamoxifen monotherapy [63]. Similarly, a clinical study including ER+ breast cancer patients showed that treatment with neoadjuvant letrozole and everolimus before surgery resulted in higher clinical response and reduced tumor proliferation compared to letrozole alone [64].
Sapanisertib (MLN0128) is an oral, potent, and highly selective ATP-competitive inhibitor of mTOR kinase that exhibits dual specificity against both mTOR complexes (mTORC1 and mTORC2). In a phase II study (NCT02049957), sapanisertib plus exemestane or fulvestrant was well tolerated and exhibited clinical benefit in postmenopausal women with pretreated everolimus-sensitive or everolimus-resistant breast cancer [65][66]. A randomized study of AZD2014 (vistusertib) in combination with fulvestrant in metastatic or advanced breast cancer (MANTA, NCT02216786) was conducted. The combination of fulvestrant and everolimus demonstrated significantly longer PFS compared to fulvestrant and vistusertib or fulvestrant alone. The trial failed to demonstrate a benefit of adding the dual mTORC1 and mTORC2 inhibitor vistusertib to fulvestrant [67].
mMTOR inhibitors were generally well tolerated in clinical trials. The most frequently observed side effects included headache, fatigue, and erythema (skin rash). In particular, the use of MTOR inhibitors was associated with a higher risk of developing hypertriglyceridemia, hypercholesterolemia, and hyperglycemia [57][68][69][70]. Future studies should take into account the improvement of clinical benefits together with reduced risk of adverse events.
-
Dual PI3K/mTOR Inhibitors
During the early developmental phases of mTOR and PI3K inhibitors, it was noted that the catalytic pocket of these two enzymes possess structural similarities, making it possible to design ATP-competitive drugs targeting both kinases simultaneously [71][72]. In particular, mTOR inhibition commonly results in the repression of a negative feedback loop, which activates the PI3K and MAPK pathways. In line with this, inhibition of both PI3K and mTOR was proposed as a good strategy to limit this compensatory mechanism [73][74]. In breast cancer, gedatolisib (PF-05212384) is a dual PI3K/mTOR inhibitor that was evaluated in combination with either docetaxel, cisplatin, or dacomitinib in triple-negative breast cancer (NCT01920061). This phase I study assessed the safety, pharmacokinetics, and pharmacodynamics of these combinations in patients with advanced cancer in order to determine the maximum tolerated dose in each combination. The cisplatin combination expansion portion was used to evaluate the anti-tumor activity of gedatolisib plus cisplatin in patients with TNBC in two separate arms. A manageable toxicity profile was observed in gedatolisib combined with docetaxel, cisplatin, or dacomitinib. Dose escalation to determine the maximum tolerated dose is still ongoing [75]. Another phase I study was conducted to assess the tolerability and clinical activity of gedatolisib in combination with either palbociclib/letrozole or palbociclib/fulvestrant in women with metastatic breast cancer (NCT02684032). This clinical trial was recently concluded; however, results are not yet available. Gedatolisib combined with either palbociclib/letrozole or palbociclib/fulvestrant showed manageable toxicity and promising antitumor activity. Further analysis on dose escalation is being completed and dose expansion is ongoing [76]. Whether the effect of this class of agents in combination with immunotherapy can lead to further clinical benefit is an open issue.
5. Rationale for Targeting Class II PI3K in Breast Cancer
Class II PI3Ks consist of three genes encoding for distinct functional isoforms: PI3K-C2α and PI3K-C2β, which are ubiquitously expressed [2][77], and PI3K-C2γ, whose expression is mainly restricted to liver [78][79]. Different from class I, class II PI3Ks act as monomers, regulating vesicle trafficking and membrane remodeling through their conserved N-terminal domain [2][80][81]. They synthetize PI(3)P on endosomes and PI(3,4)P2 at plasma membrane [79][80][82][83][84][85][86][87][88]. However, their catalytic pocket is structurally different from class I PI3K and largely unaffected by treatments with class I PI3K inhibitors [89][90][91]. Recent studies suggested that class II PI3Ks are directly involved in breast cancer progression independently of class I PI3K, opening the way for the development of new therapeutic strategies targeting this enigmatic class of PI3K in breast cancer.
PI3K-C2α is the most studied isoform, and it has been linked with breast cancer in different studies. Overexpression of the PI3K-C2α encoding gene, PIK3C2A, was found in an MCF7 cancer stem-cell side population, correlating with increased tumorigenesis in mouse models [92]. This suggests that PI3K-C2α might have a role in the early phases of cancer development. Conversely, PI3K-C2α was found to rarely be mutated in breast cancer patients on publicly available datasets, but it was observed as lost at both the mRNA and protein levels in a large cohort of breast cancer patients [93]. This study demonstrated that PI3K-C2α has kinase-independent activity by stabilizing microtubules at kinetochore during mitotic metaphase and allowing proper chromosome congression [93]. Loss of this activity was associated with increased genomic instability that led to the emergence of fast-growing clones with mitotic checkpoint defects. Therefore, low PIK3C2A expression was related to high sensitivity to paclitaxel treatment in human breast cancer patients [93]. Accordingly, development of future inhibitors targeting PI3K-C2α scaffold function in breast cancer can be beneficial in combination with microtubule-targeting drugs, i.e., paclitaxel.
Different from PI3K-C2α, PI3K-C2β isoform was found to be overexpressed in human primary breast tumors and in lymph-node metastases compared to non-neoplastic breast tissue [94]. An additional study conducted on different human breast cancer cell lines found a similar increase in PI3K-C2β levels, which was directly related to enhanced tumorigenesis and invasive abilities both in vitro and in vivo [94]. In line with this, PI(3)P produced by PI3K-C2β was shown to be involved in breast cancer migration and invasion by dismantling lamellipodia and filipodia, thus resulting in reduced cell adhesion [95][96][97]. Consistently, data from xenograft models showed that the overexpression of PI3K-C2β leads to increased cell motility and enhanced metastasis development in vivo [94].
At the current state, there is no pharmacological option to selectively target the class II PI3K that has been clinically tested. Nevertheless, given their emerging functions in many pathological processes, some efforts have been made to find effective inhibitors targeting this less investigated class of PI3K. In general, pharmacological inhibition of class II can be achieved by “off-target” activities of different class I inhibitors [98], such as PIK90, PIK124, PI-103 [99], and NVP-BEZ235 [100]. Of note, some inhibitors, such as PI701 and PI702 [98][101], have shown to be more selective for PI3K-C2β than PI3K-C2α isoform. However, a lack of selectivity for class II keeps these options far from being effective at specifically targeting class II PI3K in any pathological process. Interestingly, a subsequent study claimed the discovery of a PIK3-C2α-selective inhibitory molecule, MIPS-21335, suggesting a potential new therapeutic anti-thrombotic approach based on class II PI3K-selective targeting [102].
Taken together, recent discoveries showed that PI3K activity in cancer development and migration is not limited to PIP3 production by class I PI3K, thus highlighting the importance of class II PI3K-derived phosphoinositides. These findings suggest that pharmacological targeting of class II PI3K may lead to the development of alternative therapeutic strategies for treating breast cancer, emphasizing the need for class II PI3K-selective inhibitors in clinic.
References
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534.
- Gulluni, F.; De Santis, M.C.; Margaria, J.P.; Martini, M.; Hirsch, E. Class II PI3K Functions in Cell Biology and Disease. Trends Cell Biol. 2019, 29, 339–359.
- Hirsch, E.; Gulluni, F.; Martini, M. Phosphoinositides in cell proliferation and metabolism. Adv. Biol. Regul. 2020, 75, 100693.
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378.
- Geering, B.; Cutillas, P.R.; Nock, G.; Gharbi, S.I.; Vanhaesebroeck, B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc. Natl. Acad. Sci. USA 2007, 104, 7809–7814.
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341.
- Kok, K.; Geering, B.; Vanhaesebroeck, B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem. Sci. 2009, 34, 115–127.
- Kok, K.; Nock, G.E.; Verrall, E.A.G.; Mitchell, M.P.; Hommes, D.W.; Peppelenbosch, M.; Vanhaesebroeck, B. Regulation of p110δ PI 3-Kinase Gene Expression. PLoS ONE 2009, 4, e5145.
- Samuels, Y.; Velculescu, V. Oncogenic Mutations of PIK3CA in Human Cancers. Cell Cycle 2004, 3, 1221–1224.
- Lindhurst, M.J.; Parker, V.E.R.; Payne, F.; Sapp, J.; Rudge, S.; Harris, J.; Witkowski, A.M.; Zhang, Q.; Groeneveld, M.P.; Scott, C.E.; et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat. Genet. 2012, 44, 928–933.
- Angulo, I.; Vadas, O.; Garçon, F.; Banham-Hall, E.; Plagnol, V.; Leahy, T.R.; Baxendale, H.; Coulter, T.; Curtis, J.; Wu, C.; et al. Phosphoinositide 3-Kinase Gene Mutation Predisposes to Respiratory Infection and Airway Damage. Science 2013, 342, 866–871.
- Lucas, C.; Kuehn, H.S.; Zhao, F.; Niemela, J.; Deenick, E.K.; Palendira, U.; Avery, D.T.; Moens, L.; Cannons, J.L.; Biancalana, M.; et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 2014, 15, 88–97.
- Lucas, C.; Zhang, Y.; Venida, A.; Wang, Y.; Hughes, J.; McElwee, J.; Butrick, M.; Matthews, H.; Price, S.; Biancalana, M.; et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J. Exp. Med. 2014, 211, 2537–2547.
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635.
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 1–29.
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88.
- Dienstmann, R.; Rodon, J.; Serra, V.; Tabernero, J. Picking the Point of Inhibition: A Comparative Review of PI3K/AKT/mTOR Pathway Inhibitors. Mol. Cancer Ther. 2014, 13, 1021–1031.
- Fritsch, C.; Huang, A.; Chatenay-Rivauday, C.; Schnell, C.; Reddy, A.; Liu, M.; Kauffmann, A.; Guthy, D.; Erdmann, D.; De Pover, A.; et al. Characterization of the Novel and Specific PI3Kα Inhibitor NVP-BYL719 and Development of the Patient Stratification Strategy for Clinical Trials. Mol. Cancer Ther. 2014, 13, 1117–1129.
- Fritsch, C.; Pfister, E.; Ebel, N.; Guthy, D.; Schnell, C.; Hofmann, F. Abstract 3934: Determination of the PI3Kα selective inhibitor alpelisib mechanism of action and efficacy in ER+/PIK3CA mutant breast cancer preclinical models. Exp. Mol. Ther. 2018, 78, 3934.
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413.
- Crowder, R.J.; Phommaly, C.; Tao, Y.; Hoog, J.; Luo, J.; Perou, C.; Parker, J.S.; Miller, M.A.; Huntsman, D.G.; Lin, L.; et al. PIK3CA and PIK3CB Inhibition Produce Synthetic Lethality when Combined with Estrogen Deprivation in Estrogen Receptor–Positive Breast Cancer. Cancer Res. 2009, 69, 3955–3962.
- Juric, D.; Janku, F.; Rodón, J.; Burris, H.A.; Mayer, I.A.; Schuler, M.; Seggewiss-Bernhardt, R.; Gil-Martin, M.; Middleton, M.R.; Baselga, J.; et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor–Positive Advanced Breast Cancer. JAMA Oncol. 2019, 5, e184475.
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940.
- Moehler, M.; Shitara, K.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; Liu, T.; et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann. Oncol. 2020, 31, S1191.
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936.
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316.
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Borrego, M.R.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDKi) + aromatase inhibitor (AI): BYLieve study results. J. Clin. Oncol. 2020, 38, 1006.
- Baselga, J.; Cortes, J.; Im, S.-A.; Clark, E.; Ross, G.; Kiermaier, A.; Swain, S. Biomarker Analyses in CLEOPATRA: A Phase III, Placebo-Controlled Study of Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive, First-Line Metastatic Breast Cancer. J. Clin. Oncol. 2014, 32, 3753–3761.
- Olivero, A.G.; Heffron, T.P.; Baumgardner, M.; Belvin, M.; Ross, L.B.; Blaquiere, N.; Bradley, E.; Castanedo, G.; Derynck, M.; Do, S.; et al. Abstract DDT02-01: Discovery of GDC-0032: A beta-sparing PI3K inhibitor active against PIK3CA mutant tumors. Cancer Chem. 2013, 73.
- Juric, D.; Krop, I.; Ramanathan, R.K.; Wilson, T.R.; Ware, J.A.; Bohorquez, S.S.; Savage, H.M.; Sampath, D.; Salphati, L.; Lin, R.S.; et al. Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors. Cancer Discov. 2017, 7, 704–715.
- Baselga, J.; Dent, S.F.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Verma, S.; Wilson, T.R.; Jin, H.; et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J. Clin. Oncol. 2018, 36, LBA1006.
- Ndubaku, C.O.; Heffron, T.P.; Staben, S.T.; Baumgardner, M.; Blaquiere, N.; Bradley, E.; Bull, R.; Do, S.; Dotson, J.; Dudley, D.; et al. Discovery of 2--2-methylpropanamide (GDC-0032): A β-Sparing Phosphoinositide 3-Kinase Inhibitor with High Unbound Exposure and Robust in Vivo Antitumor Activity. J. Med. Chem. 2013, 56, 4597–4610.
- Hanker, A.; Pfefferle, A.D.; Balko, J.M.; Kuba, M.G.; Young, C.D.; Sánchez, V.; Sutton, C.R.; Cheng, H.; Perou, C.; Zhao, J.J.; et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc. Natl. Acad. Sci. USA 2013, 110, 14372–14377.
- Loibl, S.; Majewski, I.; Guarneri, V.; Nekljudova, V.; Holmes, E.; Bria, E.; Denkert, C.; Schem, C.; Sotiriou, C.; Loi, S.; et al. Corrections to “PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: Pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab”. Ann. Oncol. 2019, 30, 1180.
- Guerin, M.; Rezai, K.; Isambert, N.; Campone, M.; Autret, A.; Pakradouni, J.; Provansal, M.; Camerlo, J.; Sabatier, R.; Bertucci, F.; et al. PIKHER2: A phase IB study evaluating buparlisib in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancer. Eur. J. Cancer 2017, 86, 28–36.
- Pistilli, B.; Pluard, T.; Urruticoechea, A.; Farci, D.; Kong, A.; Bachelot, T.; Chan, S.; Han, H.S.; Jerusalem, G.; Urban, P.; et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res. Treat. 2018, 168, 357–364.
- Loibl, S.; de la Pena, L.; Nekljudova, V.; Zardavas, D.; Michiels, S.; Denkert, C.; Rezai, M.; Bermejo, B.; Untch, M.; Lee, S.C.; et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: A randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur. J. Cancer 2017, 85, 133–145.
- Jain, S.; Shah, A.N.; Santa-Maria, C.A.; Siziopikou, K.; Rademaker, A.; Helenowski, I.; Cristofanilli, M.; Gradishar, W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018, 171, 371–381.
- Barok, M.; Tanner, M.; Köninki, K.; Isola, J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011, 13, R46.
- Zhang, M.; Jang, H.; Nussinov, R. PI3K inhibitors: Review and new strategies. Chem. Sci. 2020, 11, 5855–5865.
- Hong, R.; Edgar, K.; Song, K.; Steven, S.; Young, A.; Hamilton, P.; Arrazate, A.; De La Cruz, C.; Chan, C.; Pang, J.; et al. Abstract PD4-14: GDC-0077 is a selective PI3Kalpha inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. Poster Discuss. Abstr. 2018, 78.
- Turner, N.; Dent, R.; O’Shaughnessy, J.; Kim, S.-B.; Isakoff, S.; Barrios, C.; Saji, S.; Bondarenko, I.; Nowecki, Z.; Lian, Q.; et al. 283MO Ipatasertib (IPAT) + paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered hormone receptor-positive (HR+) HER2-negative advanced breast cancer (aBC): Primary results from Cohort B of the IPATunity130 randomised phase III trial. Ann. Oncol. 2020, 31, S354–S355.
- Merlino, G.; Fiascarelli, A.; Bigioni, M.; Bressan, A.; Irrissuto, C.; Pellacani, A.; Scaltriti, M.; Binaschi, M. Abstract 2160: MEN1611, a novel α-selective PI3K inhibitor in solid tumors. Tumor Biol. 2018, 78, 2160.
- Janku, F.; Huang, H.; Treskova, I.; Pivovarcikova, K.; Call, S.; Meric-Bernstam, F.; Pesta, M.; Polivka, J. Ultra-sensitive detection of circulating tumor DNA identifies patients in high risk of recurrence in early stages melanoma. Ann. Oncol. 2019, 30, v767.
- Hansen, A.R.; Shapiro, G.; Do, K.T.; Kumar, R.; Martin-Liberal, J.; Higano, C.S.; Wisinski, K.B.; Dean, E.J.; Heath, E.I.; Rathkopf, D.E.; et al. A first in human phase I study of AZD8186, a potent and selective inhibitor of PI3K in patients with advanced solid tumours as monotherapy and in combination with the dual mTORC1/2 inhibitor vistusertib (AZD2014) or abiraterone acetate. J. Clin. Oncol. 2017, 35, 2570.
- Owusu-Brackett, N.; Zhao, M.; Akcakanat, A.; Evans, K.W.; Yuca, E.; Dumbrava, E.I.; Janku, F.; Meric-Bernstam, F. Targeting PI3Kβ alone and in combination with chemotherapy or immunotherapy in tumors with PTEN loss. Oncotarget 2020, 11, 969–981.
- Zhang, Z.; Richmond, A. The Role of PI3K Inhibition in the Treatment of Breast Cancer, Alone or Combined With Immune Checkpoint Inhibitors. Front. Mol. Biosci. 2021, 8.
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.; Patwardhan, G.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018, 29, 2232–2239.
- Hamilton, E.; Lee, A.; Swart, R.; Newton, G.; O’Connell, B.; Roberts, J.; Zhang, H.; Soliman, H. Abstract PS11-32: Mario-3 phase II study safety run-in evaluating a novel triplet combination of eganelisib (formerly IPI-549), atezolizumab (atezo), and nab-paclitaxel (nab-pac) as first-line (1L) therapy for locally advanced or metastatic triple-negative breast cancer (TNBC). Poster Sess. Abstr. 2021, 81, PS11-32.
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grădinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331.
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT Kinases in Cancer: Implications for Therapeutic Targeting. Adv. Cancer Res. 2005, 94, 29–86.
- Banerji, U.; Dean, E.J.; Pérez-Fidalgo, J.A.; Batist, G.; Bedard, P.L.; You, B.; Westin, S.N.; Kabos, P.; Garrett, M.D.; Tall, M.; et al. A Phase I Open-Label Study to Identify a Dosing Regimen of the Pan-AKT Inhibitor AZD5363 for Evaluation in Solid Tumors and inPIK3CA-Mutated Breast and Gynecologic Cancers. Clin. Cancer Res. 2017, 24, 2050–2059.
- De Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arija, J.A.A.; Shih, K.C.; Radavoi, G.D.; Xu, N.; et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin. Cancer Res. 2019, 25, 928–936.
- Mundi, P.; Sachdev, J.; McCourt, C.; Kalinsky, K. AKT in cancer: New molecular insights and advances in drug development. Br. J. Clin. Pharmacol. 2016, 82, 943–956.
- Landel, I.; Quambusch, L.; Depta, L.; Rauh, D. Spotlight on AKT: Current Therapeutic Challenges. ACS Med. Chem. Lett. 2020, 11, 225–227.
- Xing, Y.; Lin, N.U.; Maurer, M.A.; Chen, H.; Mahvash, A.; Sahin, A.; Akcakanat, A.; Li, Y.; Abramson, V.; Litton, J.; et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019, 21, 1–12.
- Wright, S.; Vasilevski, N.; Serra, V.; Rodon, J.; Eichhorn, P. Mechanisms of Resistance to PI3K Inhibitors in Cancer: Adaptive Responses, Drug Tolerance and Cellular Plasticity. Cancers 2021, 13, 1538.
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291.
- Weisner, J.; Landel, I.; Reintjes, C.; Uhlenbrock, N.; Trajkovic-Arsic, M.; Dienstbier, N.; Hardick, J.; Ladigan, S.; Lindemann, M.; Smith, S.; et al. Preclinical Efficacy of Covalent-Allosteric AKT Inhibitor Borussertib in Combination with Trametinib in KRAS-mutant Pancreatic and Colorectal Cancer. Cancer Res. 2019, 79, 2367–2378.
- Dey, N.; De, P.; Leyland-Jones, B. PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell signaling to clinical trials. Pharmacol. Ther. 2017, 175, 91–106.
- Gnant, M.; Baselga, J.; Rugo, H.S.; Noguchi, S.; Burris, H.A.; Piccart, M.; Hortobagyi, G.N.; Eakle, J.; Mukai, H.; Iwata, H.; et al. Effect of Everolimus on Bone Marker Levels and Progressive Disease in Bone in BOLERO-2. J. Natl. Cancer Inst. 2013, 105, 654–663.
- Hasskarl, J. Everolimus. Methods Mol. Biol. 2018, 211, 101–123.
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized Phase II Trial of Everolimus in Combination With Tamoxifen in Patients With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer With Prior Exposure to Aromatase Inhibitors: A GINECO Study. J. Clin. Oncol. 2012, 30, 2718–2724.
- Royce, M.; Bachelot, T.; Villanueva, C.; Özgüroglu, M.; Azevedo, S.J.; Cruz, F.M.; Debled, M.; Hegg, R.; Toyama, T.; Falkson, C.; et al. Everolimus Plus Endocrine Therapy for Postmenopausal Women With Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. JAMA Oncol. 2018, 4, 977.
- Petrossian, K.; Nguyen, D.; Lo, C.; Kanaya, N.; Somlo, G.; Cui, Y.X.; Huang, C.-S.; Chen, S. Use of dual mTOR inhibitor MLN0128 against everolimus-resistant breast cancer. Breast Cancer Res. Treat. 2018, 170, 499–506.
- Lim, B.; Potter, D.A.; Salkeni, M.A.; Silverman, P.; Haddad, T.C.; Forget, F.; Awada, A.; Canon, J.-L.; Danso, M.; Lortholary, A.; et al. Sapanisertib Plus Exemestane or Fulvestrant in Women with Hormone Receptor–Positive/HER2-Negative Advanced or Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3329–3338.
- Schmid, P.; Zaiss, M.; Harper-Wynne, C.; Ferreira, M.; Dubey, S.; Chan, S.; Makris, A.; Nemsadze, G.; Brunt, A.M.; Kuemmel, S.; et al. Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women With Hormone Receptor–Positive Metastatic Breast Cancer. JAMA Oncol. 2019, 5, 1556–1563.
- Brana, I.; Lorusso, P.; Baselga, J.; Heath, E.I.; Patnaik, A.; Gendreau, S.; Laird, A.; Papadopoulos, K. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. J. Clin. Oncol. 2010, 28, 3030.
- Funakoshi, T.; Latif, A.; Galsky, M.D. Risk of hematologic toxicities in patients with solid tumors treated with everolimus: A systematic review and meta-analysis. Crit. Rev. Oncol. 2013, 88, 30–41.
- Tabernero, J.; Rojo, F.; Calvo, E.; Burris, H.; Judson, I.; Hazell, K.; Martinelli, E.; Cajal, S.R.Y.; Jones, S.; Vidal, L.; et al. Dose- and Schedule-Dependent Inhibition of the Mammalian Target of Rapamycin Pathway With Everolimus: A Phase I Tumor Pharmacodynamic Study in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2008, 26, 1603–1610.
- Walker, E.H.; Perisic, O.; Ried, C.; Stephens, L.; Williams, R. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nat. Cell Biol. 1999, 402, 313–320.
- Yang, H.; Rudge, D.G.; Koos, J.; Vaidialingam, B.; Yang, H.; Pavletich, N.P. mTOR kinase structure, mechanism and regulation. Nat. Cell Biol. 2013, 497, 217–223.
- Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005, 8, 179–183.
- O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signaling and Activates Akt. Cancer Res. 2006, 66, 1500–1508.
- Wainberg, Z.A.; Shapiro, G.; Curigliano, G.; Leong, S.; Kristeleit, R.S.; Maqueda, M.A.; Britten, C.D.; Milella, M.; Middleton, M.R.; Olszanski, A.J.; et al. Phase I study of the PI3K/mTOR inhibitor PF-05212384 in combination with other antitumor agents. J. Clin. Oncol. 2015, 33, 2590.
- Forero-Torres, A.; Han, H.; Dees, E.C.; Wesolowski, R.; Bardia, A.; Kabos, P.; Layman, R.M.; Lu, J.M.; Kern, K.A.; Perea, R.; et al. Phase Ib study of gedatolisib in combination with palbociclib and endocrine therapy (ET) in women with estrogen receptor (ER) positive (+) metastatic breast cancer (MBC) (B2151009). J. Clin. Oncol. 2018, 36, 1040.
- Franco, I.; Margaria, J.P.J.; De Santis, M.C.; A Ranghino, A.; Monteyne, D.; Chiaravalli, M.; Pema, M.M.; Campa, C.C.; E Ratto, E.; Gulluni, F.; et al. Phosphoinositide 3-Kinase-C2α Regulates Polycystin-2 Ciliary Entry and Protects against Kidney Cyst Formation. J. Am. Soc. Nephrol. 2015, 27, 1135–1144.
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383.
- Braccini, L.; Ciraolo, E.; Campa, C.C.; Perino, A.; Longo, D.L.; Tibolla, G.; Pregnolato, M.; Cao, Y.; Tassone, B.; Damilano, F.; et al. PI3K-C2γ is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat. Commun. 2015, 6, 7400.
- Yoshioka, K.; Yoshida, K.; Cui, H.; Wakayama, T.; Takuwa, N.; Okamoto, Y.; Du, W.; Qi, X.; Asanuma, K.; Sugihara, K.; et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 2012, 18, 1560–1569.
- Valet, C.; Chicanne, G.; Severac, C.; Chaussade, C.; Whitehead, M.A.; Cabou, C.; Gratacap, M.-P.; Gaits-Iacovoni, F.; Vanhaesebroeck, B.; Payrastre, B.; et al. Essential role of class II PI3K-C2α in platelet membrane morphology. Blood 2015, 126, 1128–1137.
- Franco, I.; Gulluni, F.; Campa, C.C.; Costa, C.; Margaria, J.P.; Ciraolo, E.; Martini, M.; Monteyne, D.; De Luca, E.; Germena, G.; et al. PI3K Class II α Controls Spatially Restricted Endosomal PtdIns3P and Rab11 Activation to Promote Primary Cilium Function. Dev. Cell 2014, 28, 647–658.
- Marat, A.L.; Wallroth, A.; Lo, W.-T.; Müller, R.; Norata, G.D.; Falasca, M.; Schultz, C.; Haucke, V. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science 2017, 356, 968–972.
- Campa, C.C.; Margaria, J.P.; Derle, A.; Del Giudice, M.; De Santis, M.C.; Gozzelino, L.; Copperi, F.; Bosia, C.; Hirsch, E. Rab11 activity and PtdIns(3)P turnover removes recycling cargo from endosomes. Nat. Chem. Biol. 2018, 14, 801–810.
- Ciraolo, E.; Gulluni, F.; Hirsch, E. Methods to Measure the Enzymatic Activity of PI3Ks. Methods Enzymol. 2014, 543, 115–140.
- Gozzelino, L.; De Santis, M.C.; Gulluni, F.; Hirsch, E.; Martini, M. PI(3,4)P2 Signaling in Cancer and Metabolism. Front. Oncol. 2020, 10, 360.
- Posor, Y.; Eichhorn-Gruenig, M.; Puchkov, D.; Schöneberg, J.; Ullrich, A.; Lampe, A.; Müller, R.; Zarbakhsh, S.; Gulluni, F.; Hirsch, E.; et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nat. Cell Biol. 2013, 499, 233–237.
- Wang, H.; Lo, W.-T.; Žagar, A.V.; Gulluni, F.; Lehmann, M.; Scapozza, L.; Haucke, V.; Vadas, O. Autoregulation of Class II Alpha PI3K Activity by Its Lipid-Binding PX-C2 Domain Module. Mol. Cell 2018, 71, 343–351.e4.
- Virbasius, J.V.; Guilherme, A.; Czech, M.P. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J. Biol. Chem. 1996, 271, 13304–13307.
- Domin, J.; Pages, F.; Volinia, S.; Rittenhouse, S.E.; Zvelebil, M.J.; Stein, R.C.; Waterfield, M.D. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 1997, 326, 139–147.
- Martini, M.; Ciraolo, E.; Gulluni, F.; Hirsch, E. Targeting PI3K in Cancer: Any Good News? Front. Oncol. 2013, 3, 108.
- Zhou, J.; Wulfkuhle, J.; Zhang, H.; Gu, P.; Yang, Y.; Deng, J.; Margolick, J.B.; Liotta, L.A.; Petricoin, E.; Zhang, Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA 2007, 104, 16158–16163.
- Gulluni, F.; Martini, M.; De Santis, M.C.; Campa, C.C.; Ghigo, A.; Margaria, J.P.; Ciraolo, E.; Franco, I.; Ala, U.; Annaratone, L.; et al. Mitotic Spindle Assembly and Genomic Stability in Breast Cancer Require PI3K-C2α Scaffolding Function. Cancer Cell 2017, 32, 444–459.e7.
- Chikh, A.; Ferro, R.; Abbott, J.J.; Piñeiro, R.; Buus, R.; Iezzi, M.; Ricci, F.; Bergamaschi, D.; Ostano, P.; Chiorino, G.; et al. Class II phosphoinositide 3-kinase C2β regulates a novel signaling pathway involved in breast cancer progression. Oncotarget 2016, 7, 18325–18345.
- Domin, J.; Harper, L.; Aubyn, D.; Wheeler, M.; Florey, O.; Haskard, D.; Yuan, M.; Zicha, D. The class II phosphoinositide 3-kinase PI3K-C2β regulates cell migration by a PtdIns(3)P dependent mechanism. J. Cell. Physiol. 2005, 205, 452–462.
- Katso, R.M.; Pardo, O.; Palamidessi, A.; Franz, C.M.; Marinov, M.; De Laurentiis, A.; Downward, J.; Scita, G.; Ridley, A.J.; Waterfield, M.D.; et al. Phosphoinositide 3-Kinase C2β Regulates Cytoskeletal Organization and Cell Migration via Rac-dependent Mechanisms. Mol. Biol. Cell 2006, 17, 3729–3744.
- Maffucci, T.; Cooke, F.T.; Foster, F.M.; Traer, C.J.; Fry, M.; Falasca, M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J. Cell Biol. 2005, 169, 789–799.
- Falasca, M.; Hamilton, J.R.; Selvadurai, M.; Sundaram, K.; Adamska, A.; Thompson, P.E. Class II Phosphoinositide 3-Kinases as Novel Drug Targets. J. Med. Chem. 2016, 60, 47–65.
- Knight, Z.; Gonzalez, B.; Feldman, M.E.; Zunder, E.R.; Goldenberg, D.D.; Williams, O.; Loewith, R.; Stokoe, D.; Balla, A.; Toth, B.; et al. A Pharmacological Map of the PI3-K Family Defines a Role for p110α in Insulin Signaling. Cell 2006, 125, 733–747.
- Kong, D.; Dan, S.; Yamazaki, K.; Yamori, T. Inhibition profiles of phosphatidylinositol 3-kinase inhibitors against PI3K superfamily and human cancer cell line panel JFCR. Eur. J. Cancer 2010, 46, 1111–1121.
- Boller, D.; Doepfner, K.T.; De Laurentiis, A.; Guerreiro, A.S.; Marinov, M.; Shalaby, T.; Depledge, P.; Robson, A.; Saghir, N.; Hayakawa, M.; et al. Targeting PI3KC2beta impairs proliferation and survival in acute leukemia, brain tumours and neuroendocrine tumours. Anticancer Res 2012, 32, 3015–3027.
- Selvadurai, M.V.; Moon, M.J.; Mountford, S.J.; Ma, X.; Zheng, Z.; Jennings, I.G.; Setiabakti, N.M.; Iman, R.P.; Brazilek, R.J.; Abidin, N.A.Z.; et al. Disrupting the platelet internal membrane via PI3KC2α inhibition impairs thrombosis independently of canonical platelet activation. Sci. Transl. Med. 2020, 12, eaar8430.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
923
Revisions:
2 times
(View History)
Update Date:
30 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




