
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ricardo Figueiredo | + 965 word(s) | 965 | 2021-09-28 08:58:18 | | | |
| 2 | Beatrix Zheng | + 236 word(s) | 1201 | 2021-09-29 03:40:25 | | |
Video Upload Options
Altered smell is one of the most prevalent symptoms in acute COVID-19 infection. Although most patients recover normal neurosensory function in a few weeks, approximately one-tenth of patients report long-term smell dysfunction, including anosmia, hyposmia, parosmia and phantosmia, with a particularly notable impact on quality of life. In this complex scenario, inflammation and cellular damage may play a key role in the pathogenesis of olfactory dysfunctions and may affect olfactory signaling from the peripheral to the central nervous system.
1. Epidemiology
Accumulating evidence indicates that altered smell is one of the most prevalent symptoms in acute COVID-19 infection [1]. In self-report studies, the estimated prevalence of olfactory disorders in acute COVID-19 ranged from 5% to 85%, depending on disease severity, and seems to be higher than in other respiratory viral infections. Although most of the patients recover normal neurosensory function in a few weeks, approximately one-tenth of patients reported long-term smell dysfunction, including anosmia, hyposmia, parosmia, and phantosmia, with a particularly notable impact on quality of life [2].
Qualitative olfactory dysfunctions are often undervalued in the clinical management of COVID-19 infection and are generally underestimated in observational self-report studies. Individuals may experience a range of persistent and prolonged olfactory sequelae in PACS ( Table 1 ). Continued loss of smell after several weeks was reported in 1.7–29% of patients with COVID-19 requiring hospitalization [3][4][5][6]. Disturbing taste and smell were also prevalent after 6 months in approximately one quarter of home-isolated young adults with a milder course of the disease [7]. In a cohort of 467 patients in the United Kingdom followed up at 4–6 weeks, participants with positive SARS-CoV-2 IgM/IgG antibodies reported a significantly higher prevalence of longstanding smell loss compared to participants with a negative antibody test, with rates of full resolution of olfactory impairment of 57.7% and 72.1%, respectively [8]. In addition, female individuals were almost 2.5 times more likely to experience persistent smell loss compared to participants of the male sex, and parosmia was also significantly associated with unresolved smell loss at 4 to 6 weeks follow-up [8].
| Author | Country | Setting | Time (Days) | Population | n | Prevalence of Olfactory/Gustatory Dysfunction |
|---|---|---|---|---|---|---|
| Garrigues 2020 [3] | France | Cross sectional, single center | 110.9 | Hospitalized | 120 | Anosmia (13.3%) |
| Chopra 2020 [4] | United States | Prospective cohort, multicenter | 60 | Hospitalized | 488 | Loss of taste and/or smell (28%) |
| Rosales-Castillo 2020 [5] | Spain | Retrospective cohort, single center | 50.8 | Hospitalized | 118 | Anosmia (1.7%) |
| Jacobs 2020 [6] | United States | Prospective cohort, single center | 35 | Hospitalized | 183 | Lack of smell (9.3%) |
| Daher 2020 [9] | Germany | Prospective cohort, single center | 42 | Hospitalized | 33 | Loss of smell (12%) |
| Klein 2020 [10] | Israel | Prospective cohort, single center | 180 | Hospitalized (5.5%) and home-isolated | 112 | Smell changes (9.8%) |
| Moreno-Pérez 2021 [11] | Spain | Prospective cohort, multicenter | 112–126 | Hospitalized (58.2%) and home-isolated | 277 | Anosmia-dysgeusia (21%) |
| Seessle 2021 [12] | Germany | Prospective cohort, single center | 360 | Hospitalized (32.3%) and home-isolated | 96 | Anosmia (20.8%) |
| Tenford 2020 [13] | United States | Cross sectional, multicenter | 14–21 | Home-isolated | 270 | Loss of smell (27%) |
| Boscolo-Rizzo 2020 [14] | Italy | Cross sectional, single center | 28 | Home-isolated | 187 | Altered sense of smell or taste (51.3%) |
| Paderno 2020 [15] | Italy | Prospective cohort, multicenter | 30 | Home-isolated | 151 | Olfactory dysfunction (17.7%) |
| Valiente-De Santis 2020 [16] | Spain | Prospective cohort, single center | 84 | Home-isolated | 108 | Anosmia (9.3%) |
| Otte 2020 [17] | Germany | Cross sectional, single center | 56 | Home-isolated | 80 | Hyposmia (45.1%) |
| Blomberg 2021 [7] | Norway | Prospective cohort, multicenter | 168 | Home-isolated | 247 | Disturbed taste/smell (27%) |
2. Pathophysiogenesis of Olfactory Dysfunction
SARS-CoV-2’s route of infection basically comprises two pathways: through cell entry factors such as angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine 2 (TMPRSS2), and furin, or through an endosomal route that does not require previous cleavage of the spike protein (S). ACE2 can act as a primary receptor, and, after virus attachment, the spike protein in its surface is cleaved and dissociated by furin, after which the subunit S2 is cleaved by TMPRSS2, changing the structure of the S2 subunit, which ultimately leads to membrane fusion and viral RNA transferring to host cell cytoplasm. An alternative pathway can also be initiated by ACE2 binding and the internalization process involving clathrin and cathepsin L, and, in this case, the virus releases its genetic material directly after endocytosis, as an alternative independent from TMPRSS2 to invade cells [18].
After entering the mouth through salivary particles, the virus can infect cells in filiform and vallate papillae, lingual epithelium and taste buds, all cells that express ACE2, starting its replication, which in turn causes taste impairment [18]. Other potential targets for cell infection due to ACE2 are vascular endothelial cells and adipocytes in parotid and salivary glands. The damage in these cells affects both blood and nutritional supplies and, indirectly, it can change taste perception [18].
Upper airway mucosa has nasal goblet and ciliated cells expressing ACE2 and TMPRSS2, and these respiratory epithelium cell types may have a role in facilitating SARS-CoV-2 infection by storing viral particles [19].
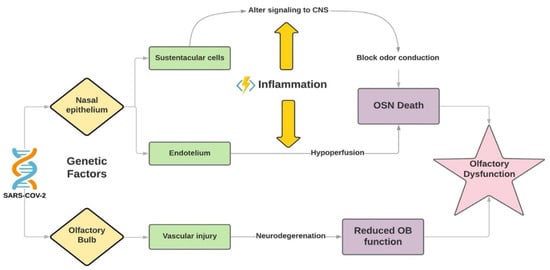
High levels of ACE2 were found in sustentacular cells of the olfactory system, which are in intimate contact with dendrites of olfactory receptor neurons, and also other olfactory epithelium cells such as ductal cells of Bowman’s gland, microvillar cells, globose and horizontal basal cells, and olfactory bulb pericytes [18][20]. It is hypothesized that infection of mesenchymal stromal and vascular cells in the nose and bulb and their subsequent inflammation affects the neuronal conduction, reduces nutritional and water supplies and, therefore, causes the death of olfactory sensory neurons (OSNs) and damage to olfactory bulb function [19] ( Figure 1 ). Although OSNs are surprisingly not an ACE2 expressing tissue, it has already been described that the spike protein can bind to neural cell receptors, possibly due to cell-to-cell transmission through tunneling nanotubes (TNTs), filamentous cellular projections that form a communication and transportation net between cells [18].
3. Smell Dysfunction in PACS
Smell dysfunction can occur in the context of various infectious viral diseases [21]. Alteration of smell can be categorized into two distinct types: quantitative and qualitative, and subcategorized in total/complete or partial/incomplete, as well as in unilateral or bilateral [21]. Quantitative loss is seen in anosmia and hyposmia, while qualitative loss is noted in parosmia and phantosmia [22].
Hyposmia was reported in a study in Padua as an isolated or more prominent symptom of SARS-CoV-2 infection, often associated with hypogeusia [23]. Hyposmia and parosmia can be persistent olfactory dysfunctions in PACS [24].
Parosmia and phantosmia are distortions in smell perception. Parosmia is a disorder in which an odor is perceived as a different smell, either pleasant—euosmia—or unpleasant—troposmia [23]. Troposmia is often referred to as a burned, foul or rotten smell [25]. In an 18-FDG PET/CT study, the activity in the secondary olfactory cortex was preserved in a patient presenting parosmia post anosmia after COVID-19 infection [25]. In another study, reduced olfactory bulb activity was associated with parosmia [25]. Parosmia and anosmia can be related, and loss of smell can evolve into parosmia in the context of SARS-CoV-2 infection [8].
Parosmia can be related to peripheral and central injuries by SARS-CoV-2, since it can affect OSNs and olfactory centers in the bulb [25]. The growth of new olfactory axons can occur in a non-organized manner and, as a consequence, it prolongs parosmia [8]. Data concerning post-infectious parosmia point to a poorer prognostic value towards the recuperation of smell ability, although olfactory training can help in the recovery of smell [8].
References
- Printza, A.; Katotomichelakis, M.; Valsamidis, K.; Metallidis, S.; Panagopoulos, P.; Panopoulou, M.; Petrakis, V.; Constantinidis, J. Smell and Taste Loss Recovery Time in COVID-19 Patients and Disease Severity. J. Clin. Med. 2021, 10, 966.
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses 2020, 45, 609–622.
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6.
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-day outcomes among patients hospitalized wed. Am. J. Physiol. Cell Physiol. 2020, 319, 945.
- Rosales-Castillo, A.; García de los Ríos, C.; Mediavilla García, J.D. Persistent symptoms after acute COVID-19 infection: Importance of follow-up. Med. Clínica Engl. Ed. 2021, 156, 35–36.
- Jacobs, L.G.; Paleoudis, E.G.; Di Bari, D.L.; Nyirenda, T.; Friedman, T.; Gupta, A.; Rasouli, L.; Zetkulic, M.; Balani, B.; Ogedegbe, C.; et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS ONE 2020, 15, e0243882.
- Blomberg, B.; Mohn, K.G.I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 23, 1–7.
- Makaronidis, J.; Firman, C.; Magee, C.G.; Mok, J.; Balogun, N.; Lechner, M.; Carnemolla, A.; Batterham, R.L. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS-CoV-2 antibodies: A community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people. BMC Infect. Dis. 2021, 21, 221.
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197.
- Hadar, K.; Kim, A.; Noam, K.; Yuval, B.; Ran, N.P.; Mordechai, M.; Sarah, I.; Masha, Y.N. Onset, duration, and persistence of taste and smell changes and other COVID-19 symptoms: Longitudinal study in Israeli patients. MedRxiv 2020.
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383.
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent symptoms in adult patients one year after COVID-19: A prospective cohort study. Clin. Infect. Dis. 2021.
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Erica, B.R.; Nathan, I.S.; Clark, F.D.; Kevin, W.G.; Heidi, L.E.; Jay, S.S.; Howard, A.S.; et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March-June 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 993–998.
- Boscolo-Rizzo, P.; Borsetto, D.; Fabbris, C.; Spinato, G.; Frezza, D.; Menegaldo, A.; Mularoni, F.; Gaudioso, P.; Cazzador, D.; Marciani, S.; et al. Evolution of Altered Sense of Smell or Taste in Patients With Mildly Symptomatic COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 729–732.
- Paderno, A.; Mattavelli, D.; Rampinelli, V.; Grammatica, A.; Raffetti, E.; Tomasoni, M.; Gualtieri, T.; Taboni, S.; Zorzi, S.; Del Bon, F.; et al. Olfactor and gustatory outcomes in COVID-19: A prospective evaluation in nonhospitalized subjects. Otolaryngol.–Head Neck Surg. 2020, 163, 1144–1149.
- Lucía, V.D.S.; Inés, P.C.; Beatriz, S.; Gracia, E.G.; Juan, D.R.M.; Antonio, P.; Ignacio, M.G.; Marcial, D.F.; Manuel, C.; Francisco, O.; et al. Clinical and immunoserological status 12 weeks after infection with COVID-19: Prospective observational study. MedRxiv 2020.
- Otte, M.S.; Eckel, H.N.C.; Poluschkin, L.; Klussmann, J.P.; Luers, J.C. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol. 2020, 140, 1032–1035.
- Okada, Y.; Yoshimura, K.; Toya, S.; Tsuchimochi, M. Pathogenesis of taste impairment and salivary dysfunction in COVID-19 patients. Jpn. Dent. Sci. Rev. 2021, 57, 111–122.
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van Den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801.
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996.
- Doty, R.L. The olfactory system and Its disorders. Semin Neurol. 2009, 29, 74–81.
- Rashid, R.A.; Alaqeedy, A.A.; Al-Ani, R.M. Parosmia Due to COVID-19 Disease: A 268 Case Series. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–8.
- Marchese-Ragona, R.; Ottaviano, G.; Piero, N.; Vianello, A.; Miryam, C. Sudden hyposmia as a prevalent symptom of COVID-19 infection. medRxiv 2020.
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021, 20, 753–761.
- Yousefi-Koma, A.; Haseli, S.; Bakhshayeshkaram, M.; Raad, N.; Karimi-Galougahi, M. Multimodality Imaging With PET/CT and MRI Reveals Hypometabolism in Tertiary Olfactory Cortex in Parosmia of COVID-19. Acad. Radiol. 2021, 28, 749–751.




