
Video Upload Options
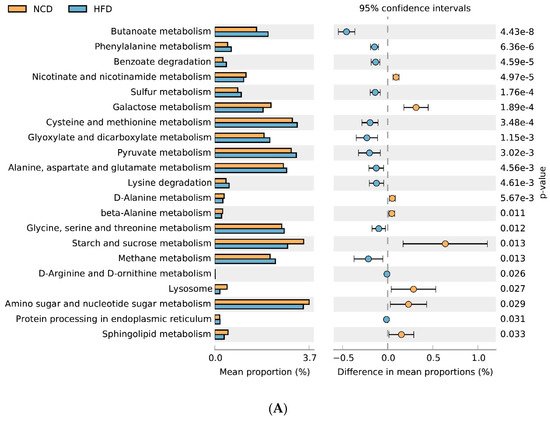
Adzuki bean consumption has many health benefits, but its effects on obesity and regulating gut microbiota imbalances induced by a high-fat diet (HFD) have not been thoroughly studied. Mice were fed a low-fat diet, a HFD, and a HFD supplemented with 15% adzuki bean (HFD-AB) for 12 weeks. Adzuki bean supplementation significantly reduced obesity, lipid accumulation, and serum lipid and lipopolysaccharide (LPS) levels induced by HFD. It also mitigated liver function damage and hepatic steatosis. In particular, adzuki bean supplementation improved glucose homeostasis by increasing insulin sensitivity. In addition, it significantly reversed HFD-induced gut microbiota imbalances. Adzuki bean significantly reduced the ratio of Firmicutes/Bacteroidetes (F/B); enriched the occurrence of Bifidobacterium, Prevotellaceae, Ruminococcus_1, norank_f_Muribaculaceae, Alloprevotella, Muribaculum, Turicibacter, Lachnospiraceae_NK4A136_group, and Lachnoclostridium; and returned HFD-dependent taxa (Desulfovibrionaceae, Bilophila, Ruminiclostridium_9, Blautia, and Ruminiclostridium) back to normal status. PICRUSt2 analysis showed that the changes in gut microbiota induced by adzuki bean supplementation may be associated with the metabolism of carbohydrates, lipids, sulfur, and cysteine and methionine; and LPS biosynthesis; and valine, leucine, and isoleucine degradation.
1. Introduction
2. Adzuki Bean Supplementation Regulated Gut Microbiota Dysbiosis
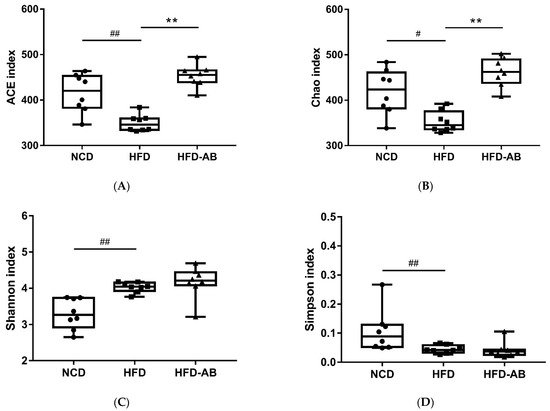
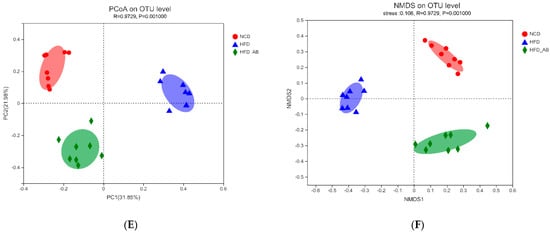
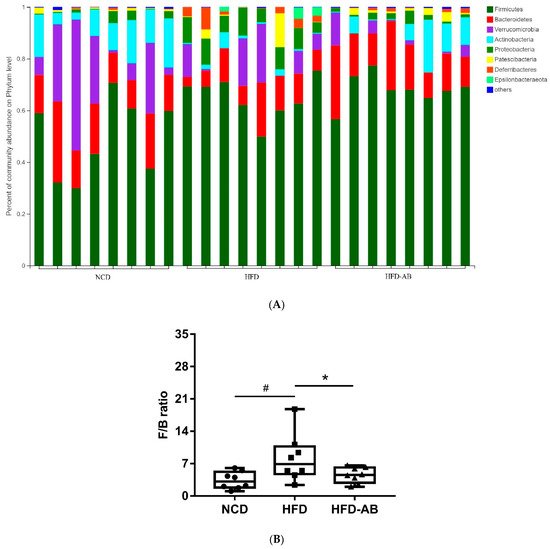
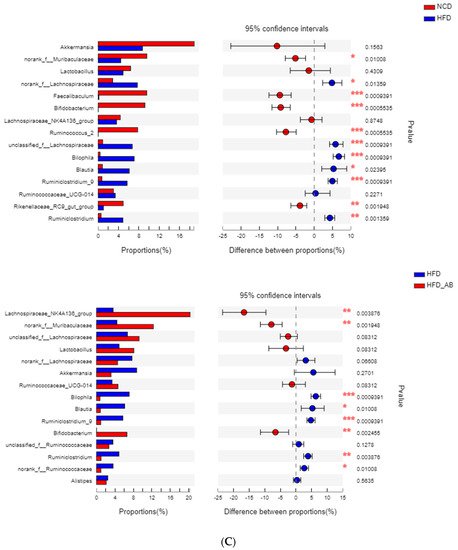
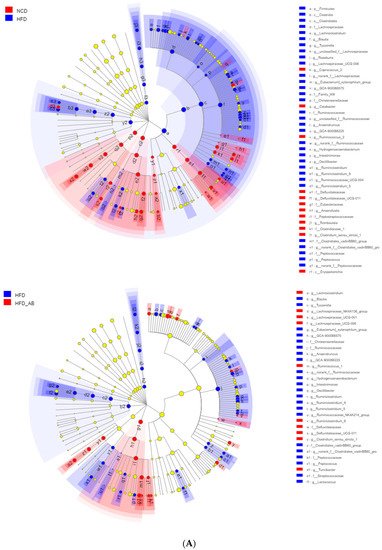
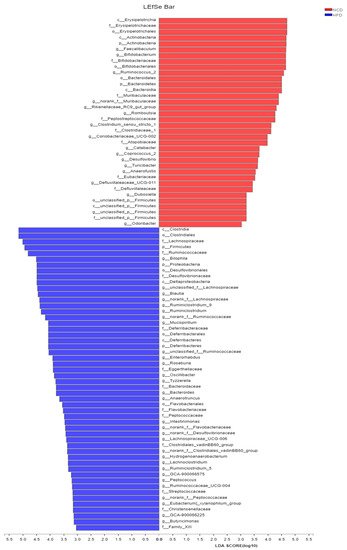
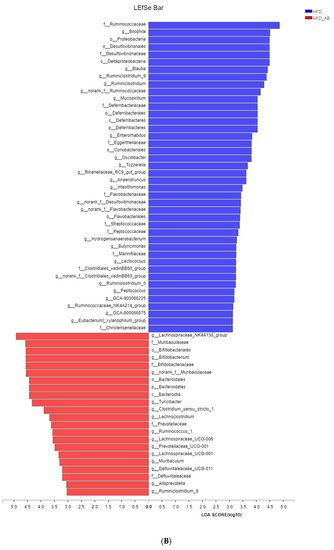
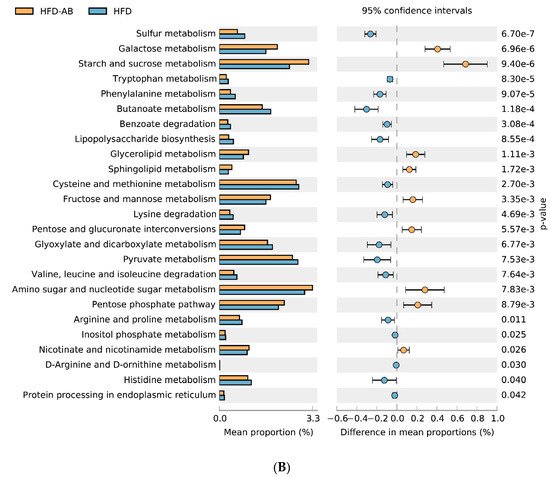
3. Gut Microbial Metabolic Functions
References
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135.
- Moreira, R.E., Jr.; de Carvalho, L.M.; Reis, D.C.D.; Cassali, G.D.; Faria, A.M.C.; Maioli, T.U.; Brunialti-Godard, A.L. Diet-induced obesity leads to alterations in behavior and gut microbiota composition in mice. J. Nutr. Biochem. 2021, 92, 108622.
- Liu, J.; Cao, J.; Li, Y.; Guo, F. Beneficial Flavonoid in Foods and Anti-obesity Effect. Food Rev. Int. 2021, 1–41.
- Moreira, R.E., Jr.; de Carvalho, L.M.; Pedersen, A.S.B.; Damasceno, S.; Maioli, T.U.; de Faria, A.M.C.; Godard, A.L.B. Interaction between high-fat diet and ethanol intake leads to changes on the fecal microbiome. J. Nutr. Biochem. 2019, 72, 108215.
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249.
- Han, K.-H.; Ohashi, S.; Sasaki, K.; Nagata, R.; Pelpolage, S.; Fukuma, N.; Reed, J.D.; Shimada, K.-I.; Kadoya, N.; Fukushima, M. Dietary adzuki bean paste dose-dependently reduces visceral fat accumulation in rats fed a normal diet. Food Res. Int. 2020, 130, 108890.
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2020, 64, 103687.
- Kitano-Okada, T.; Ito, A.; Koide, A.; Nakamura, Y.; Han, K.-H.; Shimada, K.; Sasaki, K.; Ohba, K.; Sibayama, S.; Fukushima, M. Anti-obesity role of adzuki bean extract containing polyphenols: In vivo and in vitro effects. J. Sci. Food Agric. 2012, 92, 2644–2651.
- Li, L.; Yang, T.; Liu, R.; Redden, B.; Maalouf, F.; Zong, X. Food legume production in China. Crop. J. 2017, 5, 115–126.
- Rui, L. Anti-Obesity Effects of Flavonoids and Saponins from Adzuki Bean. Ph.D. Thesis, Hong Kong Baptist University, Hong Kong, China, 2014.
- Wu, G.; Bai, Z.; Wan, Y.; Shi, H.; Huang, X.; Nie, S. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020, 101, 105456.
- Ashraf, J.; Awais, M.; Liu, L.; Khan, M.I.; Tong, L.-T.; Ma, Y.; Wang, L.; Zhou, X.; Zhou, S. Effect of thermal processing on cholesterol synthesis, solubilisation into micelles and antioxidant activities using peptides of Vigna angularis and Vicia faba. LWT 2020, 129, 109504.
- Kim, S.; Hong, J.; Jeon, R.; Kim, H.-S. Adzuki bean ameliorates hepatic lipogenesis and proinflammatory mediator expression in mice fed a high-cholesterol and high-fat diet to induce nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 90–100.
- Durak, A.; Baraniak, B.; Jakubczyk, A.; Świeca, M. Biologically active peptides obtained by enzymatic hydrolysis of Adzuki bean seeds. Food Chem. 2013, 141, 2177–2183.
- Zhao, Q.; Hou, D.; Yousaf, L.; Xue, Y.; Shen, Q. Comparison of the effects of raw and cooked adzuki bean on glucose/lipid metabolism and liver function in diabetic mice. Cereal Chem. J. 2021, 98, 1081–1090.
- Wang, J. Effect and Mechanism of Ginger on Energy Metabolism and Gut Microflora in Obese mice. Ph.D. Thesis, China Agricultural University, Beijing, China, 2019.




