
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dirk Montag | + 2219 word(s) | 2219 | 2021-09-27 03:41:42 | | | |
| 2 | Bruce Ren | -13 word(s) | 2206 | 2021-09-28 03:21:36 | | |
Video Upload Options
Molecular mechanisms underlying neuropsychiatric and neurodegenerative diseases are insufficiently elucidated. A detailed understanding of these mechanisms may help to further improve medical intervention. Recently, intellectual abilities, creativity, and amnesia have been associated with neuroplastin, a cell recognition glycoprotein of the immunoglobulin superfamily that participates in synapse formation and function and calcium signaling. Data from animal models suggest a role for neuroplastin in pathways affected in neuropsychiatric and neurodegenerative diseases. Neuroplastin loss or disruption of molecular pathways related to neuronal processes has been linked to various neurological diseases, including dementia, schizophrenia, and Alzheimer’s disease
1. Introduction

2. Neuroplastin in Neurological Diseases
2.1. Schizophrenia (SZ) and Autism Spectrum Disorder (ASD)
2.1.1. Neuroplastin Relation to Schizophrenia
2.1.2. Autism Spectrum Disorder (ASD)
2.2. Depression and Anxiety Disorder
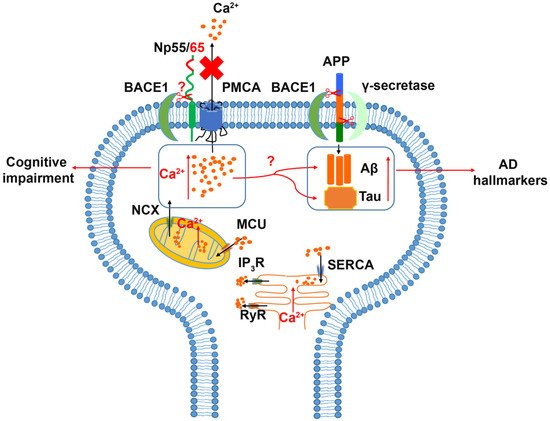
2.3. Alzheimer’s (AD) Disease
2.4. Cognition, Antero- and Retrograde Amnesia
2.5. Other Diseases Related to Neuroplastin
2.5.1. Deafness
2.5.2. Cancer
2.5.3. Various Diseases
References
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.A.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Zablotsky, B.; Black, L.I.; Maenner, M.J.; Schieve, L.A.; Danielson, M.L.; Bitsko, R.H.; Blumberg, S.J.; Kogan, M.D.; Boyle, C.A. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics 2019, 144, e20190811.
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12.
- Sloane, P.D.; Zimmerman, S.; Suchindran, C.; Reed, P.; Wang, L.; Boustani, M.; Sudha, S. The public health impact of Alzheimer’s disease, 2000–2050: Potential implication of treatment advances. Annu. Rev. Public Health 2002, 23, 213–231.
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779.
- Chong, H.Y.; Teoh, S.L.; Wu, D.B.; Kotirum, S.; Chiou, C.F.; Chaiyakunapruk, N. Global economic burden of schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 357–373.
- Rogge, N.; Janssen, J. The Economic Costs of Autism Spectrum Disorder: A Literature Review. J. Autism Dev. Disord. 2019, 49, 2873–2900.
- Baresic, A.; Nash, A.J.; Dahoun, T.; Howes, O.; Lenhard, B. Understanding the genetics of neuropsychiatric disorders: The poten-tial role of genomic regulatory blocks. Mol. Psychiatry 2020, 25, 6–18.
- Sakurai, K.; Migita, O.; Toru, M.; Arinami, T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol. Psychiatry 2002, 7, 412–415.
- Munafo, M.R.; Attwood, A.S.; Flint, J. Neuregulin 1 genotype and schizophrenia. Schizophr. Bull. 2008, 34, 9–12.
- Bernstein, H.G.; Bogerts, B. Neuregulin-1 alpha, the underestimated molecule: Emerging new roles in normal brain function and the pathophysiology of schizophrenia? Genome 2013, 56, 703–704.
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.; Holmans, P.A.; Lee, P.; Bulik-Sullivan, B.; Collier, D.A.; Huang, H.; et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427.
- Dennison, C.A.; Legge, S.E.; Pardinas, A.F.; Walters, J.T.R. Genome-wide association studies in schizophrenia: Recent advances, challenges and future perspective. Schizophr. Res. 2020, 217, 4–12.
- Jamain, S.; Quach, H.; Betancur, C.; Rastam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29.
- Yan, J.; Oliveira, G.; Coutinho, A.; Yang, C.; Feng, J.; Katz, C.; Sram, J.; Bockholt, A.; Jones, I.R.; Craddock, N.; et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol. Psychiatry 2005, 10, 329–332.
- Sudhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911.
- Sun, Y.; Yao, X.; March, M.E.; Meng, X.; Li, J.; Wei, Z.; Sleiman, P.M.A.; Hakonarson, H.; Xia, Q.; Li, J. Target Genes of Autism Risk Loci in Brain Frontal Cortex. Front. Genet. 2019, 10, 707.
- Anney, R.J.L.; Ripke, S.; Anttila, V.; J Grove, J.; Holmans, P.; Huang, H.; Klei, L.; Lee, P.H.; Medland, S.E.; Neale, B.; et al. Me-ta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 2017, 8, 21.
- Hung, A.Y.; Futai, K.; Sala, C.; Valtschanoff, J.G.; Ryu, J.; Woodworth, M.A.; Kidd, F.L.; Sung, C.C.; Miyakawa, T.; Bear, M.F.; et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J. Neurosci. 2008, 28, 1697–1708.
- Peca, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442.
- Wang, X.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108.
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260.
- Won, H.; Lee, H.R.; Gee, H.Y.; Mah, W.; Kim, J.I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like social behav-iour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012, 486, 261–265.
- Wang, X.; Christian, K.M.; Song, H.; Ming, G.L. Synaptic dysfunction in complex psychiatric disorders: From genetics to mecha-nisms. Genome Med. 2018, 10, 9.
- Penzes, P.; Buonanno, A.; Passafaro, M.; Sala, C.; Sweet, R.A. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J. Neurochem. 2013, 126, 165–182.
- Lopatina, O.L.; Malinovskaya, N.A.; Komleva, Y.K.; Gorina, Y.V.; Shuvaev, A.N.; Olovyannikova, R.Y.; Belozor, O.S.; Belova, O.A.; Higashida, H.; Salmina, A.B. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev. Neurosci. 2019, 30, 807–820.
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 2017, 390, 2327–2329.
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443.
- Paik, J. Olanzapine/Samidorphan: First Approval. Drugs 2021, 81, 1431–1436.
- LeClerc, S.; Easley, D. Pharmacological therapies for autism spectrum disorder: A review. Pharm. Ther. 2015, 40, 389–397.
- Baribeau, D.; Anagnostou, E. Novel treatments for autism spectrum disorder based on genomics and systems biology. Pharmacol. Ther. 2021, 107939.
- Doherty, J.L.; Owen, M.J. Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Med. 2014, 6, 29.
- Zheng, Z.; Zheng, P.; Zou, X. Association between schizophrenia and autism spectrum disorder: A systematic review and me-ta-analysis. Autism Res. 2018, 11, 1110–1119.
- Grant, S.G. Synaptopathies: Diseases of the synaptome. Curr. Opin. Neurobiol. 2012, 22, 522–529.
- Brose, N.; O’Connor, V.; Skehel, P. Synaptopathy: Dysfunction of synaptic function? Biochem. Soc. Trans. 2010, 38, 443–444.
- Eltokhi, A.; Janmaat, I.E.; Genedi, M.; Haarman, B.C.M.; Sommer, I.E.C. Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J. Neurosci. Res. 2020, 98, 1335–1369.
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of brain overgrowth in the first year of life in autism. JAMA 2003, 290, 337–344.
- Walker, L.; Gozzi, M.; Lenroot, R.; Thurm, A.; Behseta, B.; Swedo, S.; Pierpaoli, C. Diffusion tensor imaging in young children with autism: Biological effects and potential confounds. Biol. Psychiatry 2012, 72, 1043–1051.
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143.
- Belmonte, M.K.; Allen, G.; Beckel-Mitchener, A.; Boulanger, L.M.; Carper, R.A.; Webb, S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004, 24, 9228–9231.
- Schumann, C.M.; Bloss, C.S.; Barnes, C.C.; Wideman, G.M.; Carper, R.A.; Akshoomoff, N.; Pierce, K.; Hagler, D.; Schork, N.; Lord, C.; et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010, 30, 4419–4427.
- Gogtay, N. Cortical brain development in schizophrenia: Insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr. Bull. 2008, 34, 30–36.
- Keshavan, M.S.; Anderson, S.; Pettegrew, J.W. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J. Psychiatr. 1994, 28, 239–265.
- Mallya, A.P.; Deutch, A.Y. (Micro)Glia as Effectors of Cortical Volume Loss in Schizophrenia. Schizophr. Bull. 2018, 44, 948–957.
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385.
- Ouchi, Y.; Kubota, Y.; Kuramasu, A.; Watanabe, T.; Ito, C. Gene expression profiling in whole cerebral cortices of phencyclidine- or methamphetamine-treated rats. Mol. Brain Res. 2005, 140, 142–149.
- Sato, M.; Chen, C.C.; Akiyama, K.; Otsuki, S. Acute exacerbation of paranoid psychotic state after long-term abstinence in pa-tients with previous methamphetamine psychosis. Biol. Psychiatry 1983, 18, 429–440.
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. J. Psychiatry 1991, 148, 1301–1308.
- Saito, A.; Fujikura-Ouchi, Y.; Kuramasu, A.; Shimoda, K.; Akiyama, K.; Matsuoka, H.; Ito, C. Association study of putative pro-moter polymorphisms in the neuroplastin gene and schizophrenia. Neurosci. Lett. 2007, 411, 168–173.
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced Prepulse Inhibition as a Biomarker of Schizophrenia. Front. Behav. Neurosci. 2016, 10, 202.
- Bhattacharya, S.; Herrera-Molina, R.; Sabanov, V.; Ahmed, T.; Iscru, E.; Stober, F.; Richter, K.; Fischer, K.D.; Angenstein, F.; Gold-schmidt, J.; et al. Genetically Induced Retrograde Amnesia of Associative Memories After Neuroplastin Ablation. Biol. Psy-chiatry 2017, 81, 124–135.
- Lin, X.; Brunk, M.G.K.; Yuanxiang, P.; Curran, A.W.; Zhang, E.; Stober, F.; Goldschmidt, J.; Gundelfinger, E.D.; Vollmer, M.; Hap-pel, M.F.K.; et al. Neuroplastin expression is essential for hearing and hair cell PMCA expression. Brain Struct. Funct. 2021, 226, 1533–1551.
- Smith, M.; Spence, M.A.; Flodman, P. Nuclear and mitochondrial genome defects in autisms. Ann. N. Y. Acad. Sci. 2009, 1151, 102–132.
- Liu, Y.; Zhang, Y.; Zarrei, M.; Dong, R.; Yang, X.; Zhao, D.; Scherer, S.W.; Gai, Z. Refining critical regions in 15q24 microdeletion syndrome pertaining to autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 217–226.
- Carayol, J.; Sacco, R.; Tores, F.; Rousseau, F.; Lewin, P.; Hager, J.; Persico, A.M. Converging evidence for an association of ATP2B2 allelic variants with autism in male subjects. Biol. Psychiatry 2011, 70, 880–887.
- Blanken, L.M.; Mous, S.E.; Ghassabian, A.; Muetzel, R.L.; Schoemaker, N.K.; El Marroun, H.; van der Lugt, A.; Jaddoe, V.W.; Hof-man, A.; Verhulst, F.C.; et al. Cortical morphology in 6- to 10-year old children with autistic traits: A population-based neuroimaging study. Am. J. Psychiatry 2015, 172, 479–486.
- Desrivieres, S.; Lourdusamy, A.; Tao, C.; Toro, R.; Jia, T.; Loth, E.; Medina, L.M.; Kepa, A.; Fernandes, A.; Ruggeri, B.; et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol. Psychiatry 2015, 20, 263–274.
- Persico, A.M.; Bourgeron, T. Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends Neurosci. 2006, 29, 349–358.
- Herrera-Molina, R.; Sarto-Jackson, I.; Montenegro-Venegas, C.; Heine, M.; Smalla, K.H.; Seidenbecher, C.I.; Beesley, P.W.; Gun-delfinger, E.D.; Montag, D. Structure of excitatory synapses and GABAA receptor localization at inhibitory synapses are regulated by neuroplastin-65. J. Biol. Chem. 2014, 289, 8973–8988.
- Carrott, L.; Bowl, M.R.; Aguilar, C.; Johnson, S.L.; Chessum, L.; West, M.; Morse, S.; Dorning, J.; Smart, E.; Hardisty-Hughes, R.; et al. Absence of Neuroplastin-65 Affects Synaptogenesis in Mouse Inner Hair Cells and Causes Profound Hearing Loss. J. Neurosci. 2016, 36, 222–234.
- Li, H.; Liu, Y.; Gao, X.; Liu, L.; Amuti, S.; Wu, D.; Jiang, F.; Huang, L.; Wang, G.; Zeng, J.; et al. Neuroplastin 65 modulates anxiety- and depression-like behavior likely through adult hippocampal neurogenesis and central 5-HT activity. FEBS J. 2019, 286, 3401–3415.
- Vemula, S.K.; Malci, A.; Junge, L.; Lehmann, A.C.; Rama, R.; Hradsky, J.; Matute, R.A.; Weber, A.; Prigge, M.; Naumann, M.; et al. The Interaction of TRAF6 With Neuroplastin Promotes Spinogenesis During Early Neuronal Development. Front. Cell Dev. Biol. 2020, 8, 579513.
- Amuti, S.; Tang, Y.; Wu, S.; Liu, L.; Huang, L.; Zhang, H.; Li, H.; Jiang, F.; Wang, G.; Liu, X.; et al. Neuroplastin 65 mediates cogni-tive functions via excitatory/inhibitory synapse imbalance and ERK signal pathway. Neurobiol. Learn. Mem. 2016, 127, 72–83.
- Diefenbach, G.J.; McCarthy-Larzelere, M.E.; Williamson, D.A.; Mathews, A.; Manguno-Mire, G.M.; Bentz, B.G. Anxiety, depres-sion, and the content of worries. Depress. Anxiety 2001, 14, 247–250.
- Tiller, J.W. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31.
- Gottschalk, M.G.; Domschke, K. Genetics of generalized anxiety disorder and related traits. Dialogues Clin. Neurosci. 2017, 19, 159–168.
- Ilic, K.; Mlinac-Jerkovic, K.; Jovanov-Milosevic, N.; Simic, G.; Habek, N.; Bogdanovic, N.; Kalanj-Bognar, S. Hippocampal expres-sion of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer’s disease. J. Cell. Mol. Med. 2019, 23, 1602–1607.
- Ilic, K.; Mlinac-Jerkovic, K.; Sedmak, G.; Rosenzweig, I.; Kalanj-Bognar, S. Neuroplastin in human cognition: Review of literature and future perspectives. Transl. Psychiatry 2021, 11, 394.
- Berrocal, M.; Marcos, D.; Sepulveda, M.R.; Perez, M.; Avila, J.; Mata, A.M. Altered Ca2+ dependence of synaptosomal plasma membrane Ca2+-ATPase in human brain affected by Alzheimer’s disease. FASEB J. 2009, 23, 1826–1834.
- Mark, R.J.; Hensley, K.; Butterfield, D.A.; Mattson, M.P. Amyloid beta-peptide impairs ion-motive ATPase activities: Evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J. Neurosci. 1995, 15, 6239–6249.
- Berrocal, M.; Corbacho, I.; Vazquez-Hernandez, M.; Avila, J.; Sepulveda, M.R.; Mata, A.M. Inhibition of PMCA activity by tau as a function of aging and Alzheimer’s neuropathology. Biochim. Biophys. Acta 2015, 1852, 1465–1476.
- Arancio, A.; Ilya, B.; Berger, T.; Bouteiller, J.M.; Carrillo, M.; Disterhoft, J.; Foskett, K.; Khachaturian, A.S.; LaFerla, F.; Landfield, P.W.; et al. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a com-prehensive theory of pathogenesis. Alzheimers Dement. 2017, 13, 178–182.e117.
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharm. Sin. 2017, 38, 1205–1235.
- Luo, Y.; Bolon, B.; Kahn, S.; Bennett, B.D.; Babu-Khan, S.; Denis, P.; Fan, W.; Kha, H.; Zhang, J.; Gong, Y.; et al. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001, 4, 231–232.
- Das, B.; Yan, R. A Close Look at BACE1 Inhibitors for Alzheimer’s Disease Treatment. CNS Drugs 2019, 33, 251–263.
- Johnson, J.L.; Chambers, E.; Jayasundera, K. Application of a Bioinformatics-Based Approach to Identify Novel Putative in vivo BACE1 Substrates. Biomed. Eng. Comput. Biol. 2013, 5, 1–15.
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Piccininni, C.; Dibello, V.; Stallone, R.; Giannelli, G.; Bellomo, A.; Greco, A.; et al. BACE inhibitors in clinical development for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2018, 18, 847–857.
- Orwig, W.; Diez, I.; Bueicheku, E.; Vannini, P.; Beaty, R.; Sepulcre, J. Cortical Networks of Creative Ability Trace Gene Expression Profiles of Synaptic Plasticity in the Human Brain. Front. Hum. Neurosci 2021, 15, 694274.
- Herrera-Molina, R.; Mlinac-Jerkovic, K.; Ilic, K.; Stober, F.; Vemula, S.K.; Sandoval, M.; Milosevic, N.J.; Simic, G.; Smalla, K.H.; Goldschmidt, J.; et al. Neuroplastin deletion in glutamatergic neurons impairs selective brain functions and calcium regula-tion: Implication for cognitive deterioration. Sci. Rep. 2017, 7, 7273.
- Young, C.; Butcher, R. Propranolol for Post-Traumatic Stress Disorder: A Review of Clinical Effectiveness; CADTH Rapid Response Re-ports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020.
- Roed, A.; Brodal, B. Inhibition of sarcolemma ATPases by some membrane-stabilizing drugs. Acta Pharm. Toxicol. 1981, 48, 65–68.
- Zeng, W.Z.; Grillet, N.; Dewey, J.B.; Trouillet, A.; Krey, J.F.; Barr-Gillespie, P.G.; Oghalai, J.S.; Muller, U. Neuroplastin Isoform Np55 Is Expressed in the Stereocilia of Outer Hair Cells and Required for Normal Outer Hair Cell Function. J. Neurosci. 2016, 36, 9201–9216.
- Bortolozzi, M.; Mammano, F. PMCA2 pump mutations and hereditary deafness. Neurosci. Lett. 2018, 663, 18–24.
- Gaspar, C.; Cardoso, J.; Franken, P.; Molenaar, L.; Morreau, H.; Moslein, G.; Sampson, J.; Boer, J.M.; de Menezes, R.X.; Fodde, R. Cross-species comparison of human and mouse intestinal polyps reveals conserved mechanisms in adenomatous polyposis coli (APC)-driven tumorigenesis. Am. J. Pathol. 2008, 172, 1363–1380.
- Rodriguez-Pinto, D.; Sparkowski, J.; Keough, M.P.; Phoenix, K.N.; Vumbaca, F.; Han, D.K.; Gundelfinger, E.D.; Beesley, P.; Claffey, K.P. Identification of novel tumor antigens with patient-derived immune-selected antibodies. Cancer Immunol. Im-munother. 2009, 58, 221–234.
- Sumardika, I.W.; Chen, Y.; Tomonobu, N.; Kinoshita, R.; Ruma, I.M.W.; Sato, H.; Kondo, E.; Inoue, Y.; Yamauchi, A.; Murata, H.; et al. Neuroplastin-beta mediates S100A8/A9-induced lung cancer dissminative progression. Mol. Carcinog. 2019, 58, 980–995.
- Bajkowska, K.; Sumardika, I.W.; Tomonobu, N.; Chen, Y.; Yamamoto, K.I.; Kinoshita, R.; Murata, H.; Gede Yoni Komalasari, N.L.; Jiang, F.; Yamauchi, A.; et al. Neuroplastin beta-mediated upregulation of solute carrier family 22 member 18 antisense (SLC22A18AS) plays a crucial role in the epithelial-mesenchymal transition, leading to lung cancer cells’ enhanced motility. Biochem. Biophys. Rep. 2020, 22, 100768.
- Korthals, M.; Langnaese, K.; Smalla, K.H.; Kahne, T.; Herrera-Molina, R.; Handschuh, J.; Lehmann, A.C.; Mamula, D.; Naumann, M.; Seidenbecher, C.; et al. A complex of Neuroplastin and Plasma Membrane Ca(2+) ATPase controls T cell activation. Sci. Rep. 2017, 7, 8358.
- Choy, M.K.; Javierre, B.M.; Williams, S.G.; Baross, S.L.; Liu, Y.; Wingett, S.W.; Akbarov, A.; Wallace, C.; Freire-Pritchett, P.; Rugg-Gunn, P.J.; et al. Promoter interactome of human embryonic stem cell-derived cardiomyocytes connects GWAS re-gions to cardiac gene networks. Nat. Commun. 2018, 9, 2526.




