
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bongju KIM | + 2120 word(s) | 2120 | 2021-09-15 07:58:40 | | | |
| 2 | Jessie Wu | + 1 word(s) | 2121 | 2021-09-27 08:18:26 | | | | |
| 3 | Jessie Wu | Meta information modification | 2120 | 2021-09-27 08:18:51 | | | | |
| 4 | Felix Wu | Meta information modification | 2120 | 2021-09-28 05:01:07 | | | | |
| 5 | Felix Wu | Meta information modification | 2120 | 2021-09-28 05:02:25 | | | | |
| 6 | Felix Wu | Meta information modification | 2120 | 2021-09-28 05:03:02 | | |
Video Upload Options
While conventional dental implants focus on mechanical properties, recent advances in functional carbon nanomaterials (CNMs) accelerated the facilitation of functionalities including osteoinduction, osteoconduction, and osseointegration. The surface functionalization with CNMs in dental implants has emerged as a novel strategy for reinforcement and as a bioactive cue due to their potential for mechanical reinforcing, osseointegration, and antimicrobial properties. Numerous developments in the fabrication and biological studies of CNMs have provided various opportunities to expand their application to dental regeneration and restoration. In this review, we discuss the advances in novel dental implants with CNMs in terms of tissue engineering, including material combination, coating strategies, and biofunctionalities. We present a brief overview of recent findings and progression in the research to show the promising aspect of CNMs for dental implant application. In conclusion, it is shown that further development of surface functionalization with CNMs may provide innovative results with clinical potential for improved osseointegration after implantation.
1. Introduction
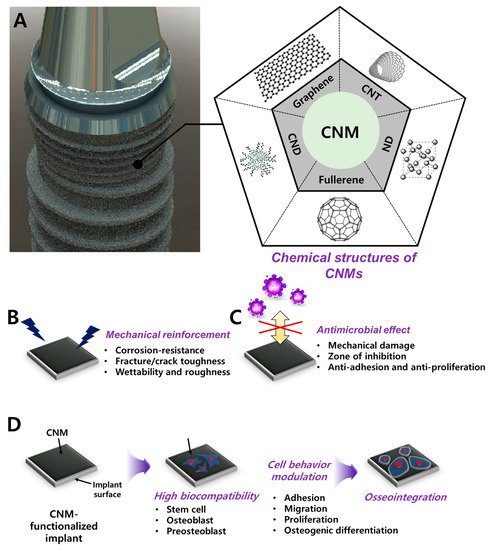
2. Biocompatibility of CNMs
| Classification of CNM | Conjugation/Combination/Modification Material | Physicochemical Advances | Osteogenic/Antimicrobial Activities | Biological Evaluation (Species) | Reference |
|---|---|---|---|---|---|
| Graphene | Zinc oxide nanocomposite coating on the acrylic tooth | - | Antimicrobial and nontoxicity on human cell | In vitro (S. mutans, HEK-293 cell) | [32] |
| G nanoplatelet coating | - | Antimicrobial effect | In vitro (S. aureus) | [61] | |
| G-doped PMMA | - | Increased bone formation indexes (NBF, BMI, LBD, BIC, BAIT, and BAOT) | In vivo (rabbit) | [62] | |
| Composite with Y-Zr ceramics | Increased density, Vickers hardness, bending strength, fracture toughness, and wettability |
- | - | [63] | |
| Graphene oxide | GO/3Y–ZrO2 composite | Reduced friction coefficient, wear rate, surface roughness. Increased wetting property. | Increased cell adhesion, proliferation, and ALP activity. | In vitro (MC3T3-E1 cell) | [64] |
| NT/GO-PEG-PEI/siRNA | - | Enhanced cell adhesion, proliferation, uptake/knockdown efficiency, osteogenic gene expression, ALP activity, collagen secretion, ECM mineralization, and in vivo osseointegration | In vitro (MC3T3-E1 cell) and in vivo (mouse) | [65] | |
| MH-loaded GO film on Ti | - | Prevention and therapeutic effect on peri-implantitis | In vivo (Beagle dog) | [66] | |
| Nano GO-coated Ti/SLA surface | Rough and irregular surface, wettability, protein adsorption | Enhanced cell proliferation, cell area, focal adhesion formation, mineralization, and osteogenic gene expression via the FAK/MAPK signaling pathway | In vitro (rBMSC) and in vivo (SD rat) | [67] | |
| MMP-2/SP-loaded GO/Ti | Enhanced roughness and wettability | MMP-2/SP delivery facilitated new bone formation | In vivo (mouse) | [68] | |
| GO/PEEK | Surface roughness and wettability | Antibacterial ability, enhanced cell viability, proliferation, ALP activity, mineralization nodule formation, osteogenic gene expression | In vitro (MG-63 cell, E. coli and S. aureus) | [69] | |
| Reduced graphene oxide | DCP-rGO composites | Controllable hybridization ratio | Cell proliferation, ALP activity, and mineralization | In vitro (MC3T3-E1 cell) | [70] |
| Dex/GO-Ti and Dex/rGO-Ti | Dex-loading capacity | Cell proliferation, osteogenic gene expression, and mineralization | In vitro (rBMSC) | [71] | |
| Dex/rGO-coated Ti13Nb13Zr | Enhanced wettability and fatigue property | Enhanced cell viability, mineralization, and osteogenic gene upregulation | In vitro (MC3T3-E1 cell) | [72] | |
| rGO/FHAp composites | Enhanced mechanical strength (GPa, MPa), ion dissolution time | Enhanced cell proliferation, ALP activity, and anti-adhesion/proliferation on bacteria | In vitro (MC3T3-E1 cell and S. mutans) | [73] | |
| rGO-coated Ti6Al4V alloy | - | Enhanced cell viability, adhesion, proliferation, mineralization nodule formation, ALP activity, and osteogenic gene expression | In vitro (MC3T3-E1 cell) | [74] | |
| Carbon nanodot | Nitrogen-doped CND/HA composite | Enhanced cell proliferation, ALP activity, mineralization nodule formation, and osteogenic gene expression. Bone regeneration in zebrafish jawbone model |
In vitro (MC3T3-E1 cell) and in vivo (zebreafish) | [75] | |
| CND/chitosan/HAp composite | Photothermal effect | Cell adhesion and osteogenesis, no lobulated neutrophils, osteocyte proliferation, tumor cell killing effects, and antibacterial effects | In vitro (rat BMSC, S. aureus and E. coli) and in vivo (mouse) | [76] | |
| Carbon nanotube | MWCNT-reinforced HAp coated Ti6Al4V implant | Cost-effective and rapid coating via electrophoresis. No microcracking, increased bond strength, and peeling resistance. |
[77] | ||
| MWCNT-reinforced HAp/316L SS implant | High corrosion protection and corrosion current density | Antibacterial effects and nanoflake morphology for enhancing bioactive potential |
In vitro (B. subtilis, S. aureus, S. flexneri and E. coli) | [78] | |
| Cu-HAp/MWCNT composite coating on 316L SS implant | High corrosion resistance | Antibacterial effect, maintained cell viability, hemolytic activity | In vitro (human osteoblast, human RBC, B. subtilis, E. coli, S. aureus, and S.mutans) | [79] | |
| Nano HAp/MWCNT coated stainless steel | Increased surface roughness | No damage on the cellular membrane and enhanced expression of osteogenic markers. | In vitro (MG-63 cell) | [80] | |
| Nanodiamond | ND/amorphous carbon composite | - | Enhanced fibronectin expression, attachment, proliferation, differentiation, calcium deposition, and ALP activity. | In vitro (EPC) | [81] |
| Icariin-functionalized ND composite | - | Icariin delivery, enhanced cell viability, particle uptake, ALP activity, calcium deposition, and osteogenic marker upregulation. | In vitro (MC3T3-E1 cell) | [82] | |
| Mg-nanodiamond composite | pH buffering, corrosion resistance, chemical passivation | Moderate cell viability | In vitro (L-929 cell) | [83] |
References
- Adya, N.; Alam, M.; Ravindranath, T.; Mubeen, A.; Saluja, B. Corrosion in titanium dental implants: Literature review. J. Indian Prosthodont. Soc. 2005, 5, 126–131.
- Teigen, K.; Jokstad, A. Dental implant suprastructures using cobalt–chromium alloy compared with gold alloy framework veneered with ceramic or acrylic resin: A retrospective cohort study up to 18 years. Clin. Oral Implant. Res. 2012, 23, 853–860.
- Karamian, E.; Motamedi, M.R.K.; Khandan, A.; Soltani, P.; Maghsoudi, S. An in vitro evaluation of novel NHA/zircon plasma coating on 316L stainless steel dental implant. Prog. Nat. Sci. 2014, 24, 150–156.
- Wang, Y.; Li, H.; Cheng, Y.; Zheng, Y.; Ruan, L. In vitro and in vivo studies on Ti-based bulk metallic glass as potential dental implant material. Mater. Sci. Eng. C 2013, 33, 3489–3497.
- Dos Santos, M.C.L.G.; Campos, M.I.G.; Line, S.R.P. Early dental implant failure: A review of the literature. Braz. J. Oral Sci. 2002, 1, 103–111.
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752.
- Tillander, J.; Hagberg, K.; Hagberg, L.; Brånemark, R. Osseointegrated titanium implants for limb prostheses attachments: Infectious complications. Clin. Orthop. Relat. Res. 2010, 468, 2781–2788.
- Tejero, R.; Anitua, E.; Orive, G. Toward the biomimetic implant surface: Biopolymers on titanium-based implants for bone regeneration. Prog. Polym. Sci. 2014, 39, 1406–1447.
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2020, 122, 26–49.
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014–2004041.
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101.
- Kang, M.S.; Lee, J.H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Nanocomposites for enhanced osseointegration of dental and orthopedic implants revisited: Surface functionalization by carbon nanomaterial coatings. J. Compos. Sci. 2021, 5, 23.
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349–e12357.
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus floor elevation by osteotome: Hand mallet versus electric mallet. A prospective clinical study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1144–1150.
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene family nanomaterials: Properties and potential applications in dentistry. Int. J. Biomater. 2018, 2018, 1–12.
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of nanomaterials in dentistry: Interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano 2015, 9, 2255–2289.
- Portelli, M.; Gatto, E.; Matarese, G.; Militi, A.; Catalfamo, L.; Gherlone, E.; Lucchese, A. Unilateral condylar hyperplasia: Diagnosis, clinical aspects and operative treatment. Eur. J. Paediatr. Dent. 2015, 16, 100–103.
- Park, J.-W.; Hanawa, T.; Chung, J.-H. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int. J. Nanomed. 2019, 14, 5697–5711.
- Rosa, A.; Kato, R.; Castro Raucci, L.; Teixeira, L.; de Oliveira, F.; Bellesini, L.; de Oliveira, P.; Hassan, M.; Beloti, M. Nanotopography drives stem cell fate toward osteoblast differentiation through α1β1 integrin signaling pathway. J. Cell. Biochem. 2014, 115, 540–548.
- Kim, E.J.; Boehm, C.A.; Mata, A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. Post microtextures accelerate cell proliferation and osteogenesis. Acta Biomater. 2010, 6, 160–169.
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323.
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189.
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212.
- Depan, D.; Shah, J.; Misra, R. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312.
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192.
- Kim, H.; Namgung, R.; Singha, K.; Oh, I.-K.; Kim, W.J. Graphene oxide–polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug. Chem. 2011, 22, 2558–2567.
- Zhu, C.; Du, D.; Lin, Y. Graphene and graphene-like 2D materials for optical biosensing and bioimaging: A review. 2D Mater. 2015, 2, 032004–032013.
- Zang, Z.; Zeng, X.; Wang, M.; Hu, W.; Liu, C.; Tang, X. Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sensor. Actuators B Chem. 2017, 252, 1179–1186.
- Kang, M.S.; Lee, J.H.; Song, S.-J.; Shin, D.-M.; Jang, J.-H.; Hyon, S.-H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Graphene oxide-functionalized nanofibre composite matrices to enhance differentiation of hippocampal neuronal cells. Mater. Adv. 2020, 1, 3496–3506.
- Shin, Y.C.; Song, S.-J.; Lee, J.H.; Park, R.; Kang, M.S.; Lee, Y.B.; Hong, S.W.; Han, D.-W. Different alignment between skeletal and smooth muscle cells on reduced graphene oxide-patterned arrays. Sci. Adv. Mater. 2020, 12, 474–480.
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2021, 25, 1–9.
- Kulshrestha, S.; Khan, S.; Meena, R.; Singh, B.R.; Khan, A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling 2014, 30, 1281–1294.
- Zhao, C.; Lu, X.; Zanden, C.; Liu, J. The promising application of graphene oxide as coating materials in orthopedic implants: Preparation, characterization and cell behavior. Biomed. Mater. 2015, 10, 015019–015028.
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980.
- Mashino, T.; Nishikawa, D.; Takahashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397.
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J. Biomed. Mater. Res. A 2014, 102, 3282–3290.
- Lobo, A.O.; Antunes, E.; Machado, A.; Pacheco-Soares, C.; Trava-Airoldi, V.; Corat, E. Cell viability and adhesion on as grown multi-wall carbon nanotube films. Mater. Sci. Eng. C 2008, 28, 264–269.
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678.
- Baik, K.Y.; Park, S.Y.; Heo, K.; Lee, K.B.; Hong, S. Carbon nanotube monolayer cues for osteogenesis of mesenchymal stem cells. Small 2011, 7, 741–745.
- Rajesh, R.; Ravichandran, Y.D. Development of new graphene oxide incorporated tricomponent scaffolds with polysaccharides and hydroxyapatite and study of their osteoconductivity on MG-63 cell line for bone tissue engineering. RSC Adv. 2015, 5, 41135–41143.
- Aversa, R.; Petrescu, R.V.; Apicella, A.; Petrescu, F.I. Nano-diamond hybrid materials for structural biomedical application. Am. J. Biochem. Biotechnol. 2016, 13, 34–41.
- Li, K.; Wang, C.; Yan, J.; Zhang, Q.; Dang, B.; Wang, Z.; Yao, Y.; Lin, K.; Guo, Z.; Bi, L.; et al. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: An in vivo study. Sci. Rep. 2018, 8, 1–10.
- Pinto, A.M.; Goncalves, I.C.; Magalhaes, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202.
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 1–8.
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564.
- Zhang, X.; Wei, C.; Li, Y.; Li, Y.; Chen, G.; He, Y.; Yi, C.; Wang, C.; Yu, D. Dose-dependent cytotoxicity induced by pristine graphene oxide nanosheets for potential bone tissue regeneration. J. Biomed. Mater. Res. A 2020, 108, 614–624.
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615.
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond. Nanotechnology 2017, 28, 252001–252028.
- Yu, S.-J.; Kang, M.-W.; Chang, H.-C.; Chen, K.-M.; Yu, Y.-C. Bright fluorescent nanodiamonds: No photobleaching and low cytotoxicity. J. Am. Chem. Soc. 2005, 127, 17604–17605.
- Paget, V.; Sergent, J.; Grall, R.; Altmeyer-Morel, S.; Girard, H.; Petit, T.; Gesset, C.; Mermoux, M.; Bergonzo, P.; Arnault, J.-C.; et al. Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines. Nanotoxicology 2014, 8, 46–56.
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004, 4, 1881–1887.
- Duch, M.C.; Budinger, G.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 2011, 11, 5201–5207.
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049.
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Ibrahim, I.; Ioannides, N.; Trzebicka, B.; Hampel, S.; Rümmeli, M.H. Graphene oxide-based drug delivery vehicles: Functionalization, characterization, and cytotoxicity evaluation. J. Nanopart. Res. 2013, 15, 1–26.
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872.
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795.
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 2013, 9, 1492–1503.
- Jacobsen, N.R.; Møller, P.; Clausen, P.A.; Saber, A.T.; Micheletti, C.; Jensen, K.A.; Wallin, H.; Vogel, U. Biodistribution of carbon nanotubes in animal models. Basic Clin. Pharmacol. Toxicol. 2017, 121, 30–43.
- Elgrabli, D.; Floriani, M.; Abella-Gallart, S.; Meunier, L.; Gamez, C.; Delalain, P.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre Toxicol. 2008, 5, 1–13.
- Lam, C.-W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134.
- Pranno, N.; La Monaca, G.; Polimeni, A.; Sarto, M.S.; Uccelletti, D.; Bruni, E.; Cristalli, M.P.; Cavallini, D.; Vozza, I. Antibacterial activity against staphylococcus aureus of titanium surfaces coated with graphene nanoplatelets to prevent peri-implant diseases. an in-vitro pilot study. Int. J. Environ. Res. Public Health 2020, 17, 1568.
- Scarano, A.; Orsini, T.; Di Carlo, F.; Valbonetti, L.; Lorusso, F. Graphene-doped poly (methyl-methacrylate) (PMMA) implants: A micro-CT and histomorphometrical study in rabbits. Int. J. Mol. Sci. 2021, 22, 1441.
- Zhang, C.; Jiang, Z.; Zhao, L.; Liu, W.; Si, P.; Lan, J. Synthesis and characterization of multilayer graphene oxide on yttria-zirconia ceramics for dental implant. J. Mater. Res. 2020, 35, 2466–2477.
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 47, 6940–6946.
- Zhang, L.; Zhou, Q.; Song, W.; Wu, K.; Zhang, Y.; Zhao, Y. Dual-functionalized graphene oxide based siRNA delivery system for implant surface biomodification with enhanced osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 34722–34735.
- Qian, W.; Qiu, J.; Liu, X. Minocycline hydrochloride-loaded graphene oxide films on implant abutments for peri-implantitis treatment in beagle dogs. J. Periodontol. 2020, 91, 792–799.
- Li, Q.; Wang, Z. Involvement of FAK/P38 signaling pathways in mediating the enhanced osteogenesis induced by nano-graphene oxide modification on titanium implant surface. Int. J. Nanomed. 2020, 15, 4659–4676.
- La, W.-G.; Jin, M.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.-S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9, 107–116.
- Ouyang, L.; Deng, Y.; Yang, L.; Shi, X.; Dong, T.; Tai, Y.; Yang, W.; Chen, Z.G. Graphene-oxide-decorated microporous polyetheretherketone with superior antibacterial capability and in vitro osteogenesis for orthopedic implant. Macromol. Biosci. 2018, 18, 1800036.
- Lee, J.J.; Shin, Y.C.; Song, S.J.; Cha, J.M.; Hong, S.W.; Lim, Y.-J.; Jeong, S.J.; Han, D.-W.; Kim, B. Dicalcium phosphate coated with graphene synergistically increases osteogenic differentiation in vitro. Coatings 2018, 8, 13.
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535.
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface modification of multipass caliber-rolled Ti alloy with dexamethasone-loaded graphene for dental applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607.
- Bai, Y.; Bai, Y.; Gao, J.; Ma, W.; Su, J.; Jia, R. Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloy. Compd. 2016, 688, 657–667.
- Li, X.; Lin, K.; Wang, Z. Enhanced growth and osteogenic differentiation of MC3T3-E1 cells on Ti6Al4V alloys modified with reduced graphene oxide. RSC Adv. 2017, 7, 14430–14437.
- Khajuria, D.K.; Kumar, V.B.; Gigi, D.; Gedanken, A.; Karasik, D. Accelerated bone regeneration by nitrogen-doped carbon dots functionalized with hydroxyapatite nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19373–19385.
- Lu, Y.; Li, L.; Li, M.; Lin, Z.; Wang, L.; Zhang, Y.; Yin, Q.; Xia, H.; Han, G. Zero-dimensional carbon dots enhance bone regeneration, osteosarcoma ablation, and clinical bacterial eradication. Bioconjug. Chem. 2018, 29, 2982–2993.
- Kaya, C.; Singh, I.; Boccaccini, A.R. Multi-walled carbon nanotube-reinforced hydroxyapatite layers on Ti6Al4V medical implants by electrophoretic deposition (EPD). Adv. Eng. Mater. 2008, 10, 131–138.
- Sivaraj, D.; Vijayalakshmi, K. Novel synthesis of bioactive hydroxyapatite/f-multiwalled carbon nanotube composite coating on 316L SS implant for substantial corrosion resistance and antibacterial activity. J. Alloy. Compd. 2019, 777, 1340–1346.
- Sivaraj, D.; Vijayalakshmi, K.; Ganeshkumar, A.; Rajaram, R. Tailoring Cu substituted hydroxyapatite/functionalized multiwalled carbon nanotube composite coating on 316L SS implant for enhanced corrosion resistance, antibacterial and bioactive properties. Int. J. Pharm. 2020, 590, 119946–119957.
- Martinelli, N.M.; Ribeiro, M.J.G.; Ricci, R.; Marques, M.A.; Lobo, A.O.; Marciano, F.R. In vitro osteogenesis stimulation via nano-hydroxyapatite/carbon nanotube thin films on biomedical stainless steel. Materials 2018, 11, 1555.
- Ivanova, L.; Popov, C.; Kolev, I.; Shivachev, B.; Karadjov, J.; Tarassov, M.; Kulisch, W.; Reithmaier, J.; Apostolova, M. Nanocrystalline diamond containing hydrogels and coatings for acceleration of osteogenesis. Diam. Relat. Mater. 2011, 20, 165–169.
- Choi, S.; Noh, S.H.; Lim, C.O.; Kim, H.-J.; Jo, H.-S.; Min, J.S.; Park, K.; Kim, S.E. Icariin-functionalized nanodiamonds to enhance osteogenic capacity in vitro. Nanomaterials 2020, 10, 2071.
- Gong, H.; Anasori, B.; Dennison, C.R.; Wang, K.; Kumbur, E.C.; Strich, R.; Zhou, J.G. Fabrication, biodegradation behavior and cytotoxicity of Mg-nanodiamond composites for implant application. J. Mater. Sci. Mater. Med. 2015, 26, 110–118.




