While conventional dental implants focus on mechanical properties, recent advances in functional carbon nanomaterials (CNMs) accelerated the facilitation of functionalities including osteoinduction, osteoconduction, and osseointegration. The surface functionalization with CNMs in dental implants has emerged as a novel strategy for reinforcement and as a bioactive cue due to their potential for mechanical reinforcing, osseointegration, and antimicrobial properties. Numerous developments in the fabrication and biological studies of CNMs have provided various opportunities to expand their application to dental regeneration and restoration. In this review, we discuss the advances in novel dental implants with CNMs in terms of tissue engineering, including material combination, coating strategies, and biofunctionalities. We present a brief overview of recent findings and progression in the research to show the promising aspect of CNMs for dental implant application. In conclusion, it is shown that further development of surface functionalization with CNMs may provide innovative results with clinical potential for improved osseointegration after implantation.
1. Introduction
Up to now, metal and metal alloy composites, including titanium, gold, stainless steel, and cobalt-chromium, have been utilized for dental implants due to their toughness, shear/fracture-resistance, and noncorrosive property [
1,
2,
3,
4,
5]. Despite their superior mechanical characteristics, low biocompatibility has become a major concern. Toxic effects caused by ions released from metallic implants induce adverse tissue reactions that lead to a low success rate in long-term clinical applications [
6]. Furthermore, metal-based dental implants need a long time to be integrated with natural bone (three to six months) owing to their non-bioactive nature that leads to low cytocompatibility and osseointegration debasement [
7,
8]. Furthermore, with recent advances in personalized and biofunctional dental implants, the conventional metal-based materials hold the faintest hope for three-dimensional (3D) printability, antibacterial properties, and drug delivery capacity [
9,
10].
While conventional dental implants focus on mechanical properties, recent advances in functional materials accelerated the facilitation of functionalities including osteoinduction, osteoconduction, and osseointegration. Osteoinduction is the process that stimulates immature cells toward preosteoblasts to start the bone healing process. Osteoconduction means that new bone grows on a material surface. Osseointegration means the facilitation of stable anchorage by bone-to-implant contact which is achieved by high osteoinduction and osteoconduction properties [
11]. Novel composite materials have been employed as a powerful tool for the alteration of physicochemical and biological properties of dental implants that allows preferred bioactivity and reducing side effects. Especially nanomaterial-based surface functionalization offers several advantages, including (i) controllable micron/nanometer-sized topography, (ii) exceptional reactivity by high surface–volume ratio, (iii) unique cell-matrix interaction, and (iv) mechanical reinforcement, which regulate bone cell behaviors and improve mechanical properties of the dental implant [
12]. Nanomaterial-functionalized surfaces highly affect cell-matrix interaction, endowing cells facilitation including survival, differentiation capability, and activity of cells. Placement of dental implants on bone tissue activates the cellular events that lead to the formation of new bone directly on the implant surface [
13]. From a clinical perspective, facilitation of bone gain, which is promoted by biochemical activities of nanomaterials, is recently highlighted for successful surgery and implant rehabilitation [
14,
15,
16]. Furthermore, tailored control of cellular behaviors offers the possibility on orthodontic treatment such as unilateral condylar hyperplasia [
17]. Therefore, nanomaterial-modified surface chemistry and topography are known to activate direct cell-matrix contact to stem cells and precursor cells, leading to higher proliferation and differentiation rate into osteogenic lineages by upregulation of osteogenic genes [
18,
19,
20].
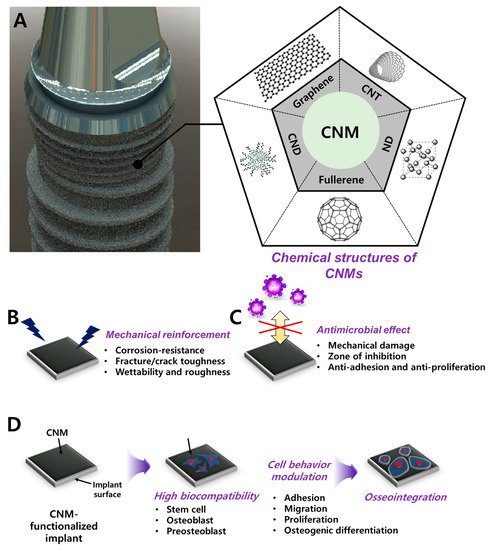
Carbon nanomaterials (CNMs) can be divided into carbon nanodot (CND), graphene (G) and its derivatives (graphene oxide; GO, reduced graphene oxide, rGO), fullerene, carbon nanotube (CNT), and nanodiamond (ND) (
Figure 1). Over the past decade, CNMs are the most highlighted nanomaterials (NMs) in various fields such as aerospace, space, electricity, electronics, and optics. CNMs have revolutionized the biomedical field with antibacterial paper [
21,
22], targeted drug delivery [
23,
24,
25], in vitro/in vivo bioimaging [
26,
27,
28], tissue engineering scaffolds [
29,
30], and dental/orthopedic implants [
31,
32,
33], with their extraordinary inherent properties.
Figure 1. Schematic diagram of CNM functionalization on dental implants for dental tissue engineering and regeneration. (A) Graphic of CNM-functionalized implants and chemical composition of the CNM family, including graphene, CNT, CND, fullerene, and ND. Enhanced properties were demonstrated, such as (B) mechanical reinforcement, (C) an antimicrobial effect, and (D) osseointegration.
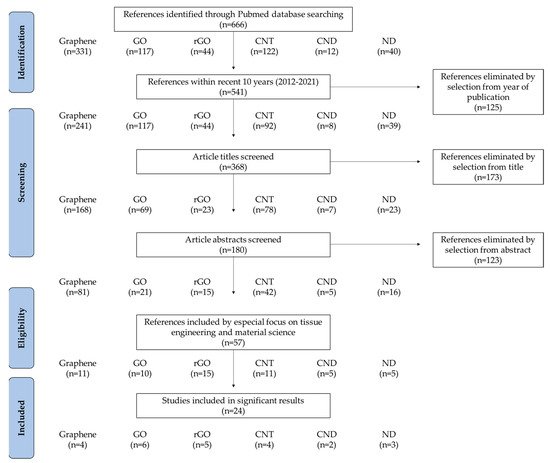
In this review, we discuss the advances in novel dental implants with CNMs in terms of tissue engineering, including material combination, coating strategies, and functionalities (Table 1). Recent studies on CNM functionalization for dental application were sorted by a PRISMA flow diagram (Figure 2). We present a brief overview of recent findings and progression in the research to show the promising aspect of CNMs for dental implant application.
Figure 2. PRISMA flow diagram denoting literature search criteria.
2. Biocompatibility of CNMs
The extensive potentials of CNM for biomedical application have been highlighted, including antibacterial [
51,
52], cell adhesion and proliferation [
53,
54], inducing osteogenic [
55,
56], osteoconduction [
57,
58], and osseointegration effects [
59]. However, biocompatibility, which often shows contradictory or inconclusive results, has issues that should be elucidated. The biocompatibility of CNMs often time-, size-, and dose-dependently works, however, it varies by raw materials, fabrication methods, and physicochemical functionalization [
60,
61,
62]. Since it is difficult to draw accurate conclusions, we intend to provide guidelines for later studies by comparing relevant studies.
GO’s dose-dependent cytotoxicity on bone marrow-derived stem cells (BMSCs) and assessed toxicity mechanisms were investigated [
63]. GO significantly inhibited cell viability at ≥2.5 µg/mL by interrupting membrane integrity. At the same concentration, cell apoptosis was one-and-a-half-fold increased but did not hinder the cell proliferation cycle significantly. Furthermore, ≥2.5 µg/mL of GO induced intracellular ROS generation, inducing ROS-associated damage, and caused cell dysfunction which was assessed by mitochondria membrane potential (MMP) loss. Western blotting showed upregulation of Cleaved Caspase-3, LC3-II/I, and Beclin-1 and downregulation of Bcl-2 and Caspase-3, indicating that GO-mediated cytotoxicity is related to mitochondrial autophagy and triggering cellular apoptosis. The hemolytic and cytotoxic effects of GO, which are synthesized in various methods, showed varying results according to their sizes, particulate states, surface charges, and oxygen contents [
64]. Hemolysis and morphologies of red blood cells (RBCs), intracellular ROS generation, and fibroblast viability were significantly different according to the fabrication methods, suggesting that the particulate state of G materials has a profound impact on cytotoxicity. The cytotoxicity and genotoxicity of different CNMs are proven to be material-specific and cell-specific with a general trend for biocompatibility (ND > carbon powder > MWCNT > SWCNT > fullerene) [
50]. For example, macrophages are more cytotoxic than neuroblastoma cells, and CNT and MWCNT tend to cause DNA damage in mouse embryonic stem cells by ROS generation [
50]. NDs possess minimal cytotoxicity because their chemical inertness does not release any toxic chemicals and round morphologies [
65]. Carboxylated NDs were shown to not exhibit cytotoxicity or genotoxicity on human cell lines including liver, kidney, intestine, and lung cell lines, which are major accumulation organs after the nanoparticles are injected [
66]. On the other hand, fullerene shows significant cytotoxicity mainly contributing to ROS generation. Fullerenes under ambient water conditions can generate superoxide anions that are responsible for membrane damage and subsequent cell death [
67].
For clinical usages including drug delivery, bioimaging, biosensing, and other theragnostic applications, in vivo toxicity of CNMs has been intensively studied. To understand the potential threat of CNMs in the body, biodistribution and accumulation mechanisms should be elucidated. The accumulation of GO in mouse lung induced oxidative stress by an increase of mitochondrial respiration and activated inflammatory and apoptotic pathways [
68]. On the other hand, surface functionalization and chemical modification have been introduced to enhance the biocompatibility and biofunctionality of G materials [
69,
70,
71]. The PEGlyated GO and rGO were developed for oral and intraperitoneal (i.p.) injection, and the biodistribution was investigated [
72]. After seven days, oral administration could not be adsorbed by organs and rapidly excreted, however, i.p.-administered PEGlyated GO and rGO were accumulated most highly in the liver and spleen but were finally engulfed by phagocytes in size- and surface coating-related manner. The results indicated that no significant toxicity was found in serum biochemistry, complete blood panel test, and histological analysis, indicating that PEGylation can facilitate biocompatibility of G materials. In a similar study, intravenous (i.v.) injected G quantum dots (GQDs) did not exhibit to vital organs of rats, although slight reduction of platelets and monocyte and eosinophil fractions occurred, which were soon normalized [
73]. After respiratory administration, CNT remains in the lungs for months or even years and is eliminated through the gastrointestinal (GI) tract. It does not cross the pulmonary barrier or get absorbed in the GI tract [
74,
75]. A single intratracheal instillation of SWCNT triggered epithelial granulomas and interstitial inflammation, developing peribronchial inflammation and necrosis [
76].
Table 1. Recent studies on CNMs for dental implant application.
| Classification of CNM |
Conjugation/Combination/Modification Material |
Physicochemical Advances |
Osteogenic/Antimicrobial Activities |
Biological Evaluation (Species) |
Reference |
| Graphene |
Zinc oxide nanocomposite coating on the acrylic tooth |
- |
Antimicrobial and nontoxicity on human cell |
In vitro (S. mutans, HEK-293 cell) |
[32] |
| G nanoplatelet coating |
- |
Antimicrobial effect |
In vitro (S. aureus) |
[77] |
| G-doped PMMA |
- |
Increased bone formation indexes (NBF, BMI, LBD, BIC, BAIT, and BAOT) |
In vivo (rabbit) |
[78] |
| Composite with Y-Zr ceramics |
Increased density, Vickers hardness,
bending strength, fracture toughness, and wettability |
- |
- |
[79] |
| Graphene oxide |
GO/3Y–ZrO2 composite |
Reduced friction coefficient, wear rate, surface roughness. Increased wetting property. |
Increased cell adhesion, proliferation, and ALP activity. |
In vitro (MC3T3-E1 cell) |
[80] |
| NT/GO-PEG-PEI/siRNA |
- |
Enhanced cell adhesion, proliferation, uptake/knockdown efficiency, osteogenic gene expression, ALP activity, collagen secretion, ECM mineralization, and in vivo osseointegration |
In vitro (MC3T3-E1 cell) and in vivo (mouse) |
[81] |
| MH-loaded GO film on Ti |
- |
Prevention and therapeutic effect on peri-implantitis |
In vivo (Beagle dog) |
[82] |
| Nano GO-coated Ti/SLA surface |
Rough and irregular surface, wettability, protein adsorption |
Enhanced cell proliferation, cell area, focal adhesion formation, mineralization, and osteogenic gene expression via the FAK/MAPK signaling pathway |
In vitro (rBMSC) and in vivo (SD rat) |
[83] |
| MMP-2/SP-loaded GO/Ti |
Enhanced roughness and wettability |
MMP-2/SP delivery facilitated new bone formation |
In vivo (mouse) |
[84] |
| GO/PEEK |
Surface roughness and wettability |
Antibacterial ability, enhanced cell viability, proliferation, ALP activity, mineralization nodule formation, osteogenic gene expression |
In vitro (MG-63 cell, E. coli and S. aureus) |
[85] |
| Reduced graphene oxide |
DCP-rGO composites |
Controllable hybridization ratio |
Cell proliferation, ALP activity, and mineralization |
In vitro (MC3T3-E1 cell) |
[86] |
| Dex/GO-Ti and Dex/rGO-Ti |
Dex-loading capacity |
Cell proliferation, osteogenic gene expression, and mineralization |
In vitro (rBMSC) |
[87] |
| Dex/rGO-coated Ti13Nb13Zr |
Enhanced wettability and fatigue property |
Enhanced cell viability, mineralization, and osteogenic gene upregulation |
In vitro (MC3T3-E1 cell) |
[88] |
| rGO/FHAp composites |
Enhanced mechanical strength (GPa, MPa), ion dissolution time |
Enhanced cell proliferation, ALP activity, and anti-adhesion/proliferation on bacteria |
In vitro (MC3T3-E1 cell and S. mutans) |
[89] |
| rGO-coated Ti6Al4V alloy |
- |
Enhanced cell viability, adhesion, proliferation, mineralization nodule formation, ALP activity, and osteogenic gene expression |
In vitro (MC3T3-E1 cell) |
[90] |
| Carbon nanodot |
Nitrogen-doped CND/HA composite |
|
Enhanced cell proliferation, ALP activity, mineralization nodule formation, and osteogenic gene expression.
Bone regeneration in zebrafish jawbone model |
In vitro (MC3T3-E1 cell) and in vivo (zebreafish) |
[91] |
| CND/chitosan/HAp composite |
Photothermal effect |
Cell adhesion and osteogenesis, no lobulated neutrophils, osteocyte proliferation, tumor cell killing effects, and antibacterial effects |
In vitro (rat BMSC, S. aureus and E. coli) and in vivo (mouse) |
[92] |
| Carbon nanotube |
MWCNT-reinforced HAp coated Ti6Al4V implant |
Cost-effective and rapid coating via electrophoresis.
No microcracking, increased bond strength, and peeling resistance. |
|
|
[93] |
| MWCNT-reinforced HAp/316L SS implant |
High corrosion protection and corrosion current density |
Antibacterial effects and
nanoflake morphology for enhancing bioactive potential |
In vitro (B. subtilis, S. aureus, S. flexneri and E. coli) |
[94] |
| Cu-HAp/MWCNT composite coating on 316L SS implant |
High corrosion resistance |
Antibacterial effect, maintained cell viability, hemolytic activity |
In vitro (human osteoblast, human RBC, B. subtilis, E. coli, S. aureus, and S.mutans) |
[95] |
| Nano HAp/MWCNT coated stainless steel |
Increased surface roughness |
No damage on the cellular membrane and enhanced expression of osteogenic markers. |
In vitro (MG-63 cell) |
[96] |
| Nanodiamond |
ND/amorphous carbon composite |
- |
Enhanced fibronectin expression, attachment, proliferation, differentiation, calcium deposition, and ALP activity. |
In vitro (EPC) |
[97] |
| Icariin-functionalized ND composite |
- |
Icariin delivery, enhanced cell viability, particle uptake, ALP activity, calcium deposition, and osteogenic marker upregulation. |
In vitro (MC3T3-E1 cell) |
[98] |
| Mg-nanodiamond composite |
pH buffering, corrosion resistance, chemical passivation |
Moderate cell viability |
In vitro (L-929 cell) |
[99] |
This entry is adapted from the peer-reviewed paper 10.3390/ma14175104