Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Aziz | + 2835 word(s) | 2835 | 2021-09-22 08:14:39 | | | |
| 2 | Rita Xu | Meta information modification | 2835 | 2021-09-27 05:33:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aziz, M. Liquid Hydrogen. Encyclopedia. Available online: https://encyclopedia.pub/entry/14556 (accessed on 07 February 2026).
Aziz M. Liquid Hydrogen. Encyclopedia. Available at: https://encyclopedia.pub/entry/14556. Accessed February 07, 2026.
Aziz, Muhammad. "Liquid Hydrogen" Encyclopedia, https://encyclopedia.pub/entry/14556 (accessed February 07, 2026).
Aziz, M. (2021, September 25). Liquid Hydrogen. In Encyclopedia. https://encyclopedia.pub/entry/14556
Aziz, Muhammad. "Liquid Hydrogen." Encyclopedia. Web. 25 September, 2021.
Copy Citation
Decarbonization plays an important role in future energy systems for reducing greenhouse gas emissions and establishing a zero-carbon society. Hydrogen is believed to be a promising secondary energy source (energy carrier) that can be converted, stored, and utilized efficiently, leading to a broad range of possibilities for future applications.
liquid hydrogen
ortho- to para-hydrogen
liquefaction
storage
1. Introduction
The increase in atmospheric greenhouse gas (GHGs) concentrations has raised concern regarding climate change, which is currently regarded as the largest global issue that needs to be urgently mitigated. In 2019, the total carbon emitted from the energy-related sector was approximately 33 Gt-CO2 [1], approximately 87% of the total globally emitted CO2. Moreover, this value is predicted to increase due to increasing global energy consumption. The Kyoto Protocol [2], which was signed by 192 countries and entered into force in 2005, was an organized global effort to combat climate change. This effort was followed by the Paris Agreement [3] in 2015, which aimed to limit the global average temperature increase to under 2 °C while actively attempting to limit this increase to 1.5 °C. The strategy to establish a low-carbon energy system is believed to be crucial for reducing GHG emissions. Several evident efforts have been proposed, including reducing fossil fuel consumption, increasing the renewable energy share, and decreasing primary energy intensity by improving energy efficiency [4][5]. The energy transition considering a strong motivation for fuel decarbonization and an increase in renewable energy share must be taken seriously for establishing a carbon-free society. Japan and China have committed to achieving their carbon neutrality targets by 2050 and 2060, respectively [6].
Recently, the COVID-19 pandemic has caused several changes in systems, including the energy system. Lower energy demand during the pandemic has resulted in the reduction of fossil fuel consumption, especially coal [7], which was running to half of its capacity by 2020. In addition, the increasing share of renewable energy sources has also cumulatively added a positive effect on the global energy transition trend [8]. However, energy transition also faces several challenges, including the storage of renewable energy sources and energy balancing following the fluctuation of renewable energy sources [4].
In terms of energy transition, hydrogen is predicted to have a more significant and pivotal role in the future, owing to its characteristics and applicability. Hydrogen is a carbon-free fuel; hence, its oxidation leads to CO2-free utilization, leading to the actualization of fuel decarbonization. Hydrogen is the most abundant element on Earth, although it is naturally available in its oxidized state (water). It can be produced from primary energy sources through various conversion technologies, including thermochemical, electrochemical, and biological routes. In addition, hydrogen is convertible to and from other secondary energy sources, such as electricity and heat, leading to possible mutual conversion among these secondary energy sources. Hydrogen utilization covers a broad range of oxidation technologies, including turbine combustion, internal combustion engines, fuel cells, and fuel mixing [9][10]. Hydrogen can also be adopted as an effective energy storage system, such as batteries. Compared to conventional batteries, which have characteristics of self-discharge and capacity degradation following the storage period and cycle, hydrogen can store the energy for a longer period, while maintaining its high energy density.
The massive future hydrogen deployment is expected to establish the hydrogen economy, in which hydrogen can be economically competitive. This massive deployment demands a broad range of hydrogen storage and transportation, ranging from small scale (e.g., vehicles) to large scale (e.g., power generation). Hydrogen is the lightest substance in this universe, with a density of 0.081 kg/m3 at 27 °C and 1 atm. Hydrogen has an excellent gravimetric energy density with a lower heating value (LHV) of 118.8 MJ/kg, but it possesses a very low volumetric energy density of approximately 3 Wh/L at ambient conditions (temperature and pressure of 20 °C and 1 atm, respectively) [11]. These characteristics pose the largest challenge in hydrogen utilization; therefore, developing and adopting an effective storage method for hydrogen is crucial. In general, hydrogen can be stored through different storage technologies, including compression, liquefaction, adsorption, hydrides, and reformed fuels. Selecting appropriate technologies to store hydrogen is influenced by its application, transportation mode, storage period, and other conditions [12].
Among these hydrogen storage systems, liquid hydrogen is considered promising in terms of both gravimetric and volumetric hydrogen densities, high hydrogen purity, and the possibility for low-pressure storage [13]. Liquid hydrogen was initially produced in 1898, and its application as a rocket fuel was adopted at the beginning of the 1950s [14]. As the demand for aerospace and other applications is increasing, the production of liquid hydrogen also increases. Moreover, the rapid growth of various hydrogen applications, including fuel-cell-based power generators and vehicles, demand high purity of hydrogen on a large scale [14], which can be provided by liquid hydrogen-based storage and transportation. Additionally, liquid hydrogen is considered to be the most feasible storage and distribution method to facilitate the demand for mobility-based hydrogen considering economy, energy density, and technical issues [15].
Figure 1 shows the basic supply chain of liquid hydrogen, covering hydrogen production, liquefaction, transportation, distribution, and utilization. Hydrogen can be produced from various primary energy sources, including conventional fossil fuels, renewable energy sources, and surplus energy (heat and electricity). This conversion can be conducted through electrochemical (electrolysis), thermochemical (gasification, pyrolysis, steam reforming, and chemical looping) [16], and biological processes (fermentation, biophotolysis, and microbial electrolysis) [17]. The produced hydrogen is then liquefied before being loaded for transportation. Various transportation options are available, such as sea tankers, trucks, and railway tankers. Transportation covers long-distance international shipping and domestic distribution. In the utilization sites, liquid hydrogen is unloaded and regasified before being distributed to small sale consumers.
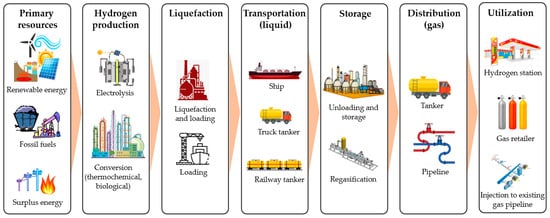
Figure 1. Basic liquid hydrogen supply chain, covering hydrogen production, liquefaction, transportation, storage, transportation, and utilization.
However, hydrogen liquefaction is an energy-intensive process. In addition, because of the extremely low temperature of −253 °C, handling and transporting liquid hydrogen requires advanced technologies and careful handling to minimize hydrogen loss and hazardous risks. Several technologies for liquefying gaseous hydrogen have been developed, including storage and transportation.
2. Hydrogen Characteristics
2.1. Hydrogen Properties
Hydrogen is the simplest substance (one proton, one electron, and no neutron), non-toxic, and has no color, odor, or taste. In addition, at ambient conditions (temperature and pressure of 20 °C and 1 atm, respectively), the hydrogen molecule is extremely small (van der Waals radius of 120 pm) and about 14 times lighter than air at 2.016 g/mol, and has a high diffusion rate (0.61 cm2/s) and buoyancy [12]. The bombardment of neutrons with hydrogen leads to the formation of isotopes, including deuterium and tritium, which are radioactive and are utilized in many nuclear devices [18]. The flashpoint (the temperature at which the fuel generates sufficient vapor amount to facilitate a flame at its surface when the ignition source exists) of hydrogen is –231 °C, which is the lowest compared to other fuels. As the flashpoint indicates easy fuel combustion, the very low flashpoint of hydrogen is advantageous because of the possibility of a simpler system to ignite and combust hydrogen [19]. Table 1 lists the physical properties of hydrogen.
Table 1. Physical properties of hydrogen.
| Properties | Value | Ref. |
|---|---|---|
| Molecular weight (g/mol) | 2.016 | [18][20] |
| Lower heating value (MJ/kg) | 118.8 | [18] |
| Higher heating value (MJ/kg) | 143 | [18] |
| Viscosity at 25 °C (cP) | 0.000892 | [20] |
| Boiling temperature at 1 atm (°C) | −253 | [18][20] |
| Melting temperature (°C) | −259 | [18][20] |
| Critical temperature (°C) | −240 | [20] |
| Critical pressure (MPa) | 1.3 | [20] |
| Density of gaseous hydrogen at 0 °C (kg/m3) | 0.08987 | [20] |
| Density of liquid hydrogen at −253 °C (kg/m3) | 70.85 | [20] |
| Density of solid hydrogen at −259 °C (kg/m3) | 858 | [20] |
| Critical density (kg/m3) | 31.2 | [20] |
| Heat capacity of gaseous hydrogen at 0 °C (kJ/kg·°C) | 14.3 | [20] |
| Heat capacity of liquid hydrogen at −256 °C (kJ/kg·°C) | 8.1 | [20] |
| Heat capacity of solid hydrogen at −259.8 °C (kJ/kg·°C) | 2.63 | [20] |
| Heat of vaporization at −253 °C (kJ/kg) | 447 | [20] |
| Heat of fusion at −259 °C (kJ/kg) | 58 | [20] |
| Thermal conductivity at 25 °C (W/cm·K) | 0.018 | [20] |
| Ionization energy (eV) | 13.5989 | [18] |
| Liquid-to-gas expansion ratio at atmospheric condition | 1:848 | [18] |
| Adiabatic flame temperature (°C) | 2107 | [18] |
| Research octane number (RON) | >130 | [18] |
| Thermal conductivity (20 °C, 1 atm) (W/m·K) | 0.1825 | [21] |
| Specific gravity of gas hydrogen at 20 °C and 1 atm | 0.0696 | [22] |
| Specific gravity of liquid hydrogen at −253 °C and 1 atm | 0.0710 | [22] |
| Latent heat of vaporization (kJ/kg) | 461 |
Figure 2 shows the correlation between hydrogen density and temperature at specific pressures. At ambient pressure (1 atm), hydrogen liquifies at a temperature of −253 °C, leading to a significant increase in its density. Hydrogen has a critical temperature and pressure of −240 °C and 1.3 MPa, respectively. Increasing the pressure to 1 MPa leads to an increase in the boiling point, although a similar graph is obtained at 0.1 MPa. When the pressure is increased further and higher than its critical point, different trends of graphs are shown because there is no latent heat under these supercritical conditions.
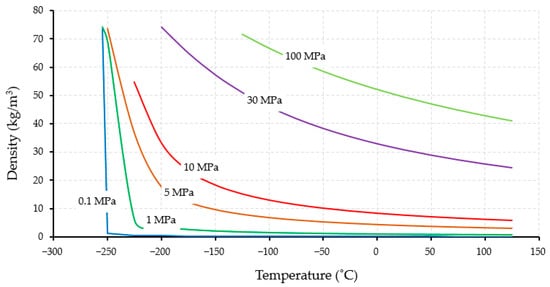
Figure 2. Correlation of hydrogen density and temperature under different pressure [23].
2.2. Comparison of Hydrogen Storage
Figure 3 shows some possible hydrogen storage options, including compression, liquefaction, hydrides, and adsorption. In physical storage, hydrogen can be stored through compression and liquefaction in the form of compressed, liquid, cryo-compressed, and slush hydrogen. In addition, chemical storage converts a broad range of materials to bind or react with hydrogen. These include hydrides (metal, interstitial metal, complex, and chemical hydrides), liquid organic hydrogen carriers (LOHC), reformed organic fuels, and hydrolysis. Among these, compression and metal hydrides are considered efficient methods for small- to medium-scale hydrogen storage [24]. Integrating large-scale energy storage into the electrical grid has the potential to solve grid problems, including the fluctuation of renewable energy [25] and storage of surplus energy.
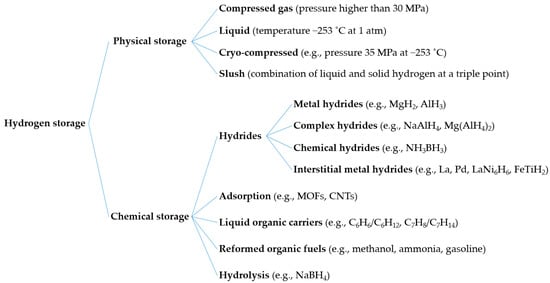
Figure 3. Hydrogen storage options, including physical and chemical storages.
Table 2 lists the characteristics comparison of several representative hydrogen storage methods, including compressed hydrogen, metal hydride, LOHC, liquid hydrogen, and liquid ammonia. These four methods are selected due to their hydrogen storage density, technological maturity, and no involvement of carbon during hydrogen utilization.
| Properties | Compressed Hydrogen | Metal Hydride (MgH2-10wt%Ni) | Liquid Organic (C7H8/C7H14) | Liquid Hydrogen |
Liquid Ammonia |
|---|---|---|---|---|---|
| Density (kg/m3) | 39 (69 MPa, 25 °C) | 1,450 | 769 (1 atm, 20 °C) | 70.9 (1 atm, −253 °C) | 682 (1 atm, −33.33 °C) |
| Boiling point (°C) | −253 | - | 101 | −253 | −33.33 |
| Gravimetric hydrogen density (wt%) | 100 | 7.10 | 6.16 | 100 | 17.8 |
| Volumetric hydrogen density (kg-H2/m3) | 42.2 | 47.1 | 70.9 | 120.3 | |
| Hydrogen release temp. (°C) | - | 250 | 200–400 | −253 | 350–900 |
| Regeneration temp. (°C) | - | - | 100–200 | - | 400–600 |
| Enthalpy change to release hydrogen (kJ/mol-H2) | - | 118 | 67.5 | 0.899 | 30.6 |
Compression is the conventional and easiest way to store hydrogen. The hydrogen density stored in its compressed form depends strongly on the storage pressure. Typically, at pressure of 10 MPa, the volumetric density of the storage hydrogen is 7.8 kg-H2/m3 (temperature of 20 °C). It increases to 39 kg-H2/m3 when the pressure is increased to approximately 69 MPa. Compressed hydrogen is adopted in many applications, including vehicles, hydrogen refueling stations, and other industrial purposes. To achieve a high density, advanced materials for vessels are required, such as carbon fiber and glass fiber-reinforced plastics. However, due to manufacturing limitations, the vessel size is also limited. In addition, because of its high pressure, the permeation of hydrogen gas (permeated hydrogen gas amount) to the vessel wall becomes larger [30] leading to higher risk of accelerated embrittlement.
Hydrides for hydrogen storage include metal, complex, chemical, and interstitial metal hydrides. Metal hydrides are intermetallic compounds formed through a combination of stable hydride-forming elements and unstable-hydride-forming elements. Common metal hydrides include MgH2, AlH2, LaNi5, and Mn2Zn [31]. Metal hydrides have the benefits of absorption and desorption at constant pressure, moderate temperature operation, and stability and safety during storage (possibility of long-term storage) [32]. However, metal hydrides also face several challenges, including limited hydrogen storage, limited reversibility, packing limitation, thermal management, and heat demand during desorption to release hydrogen [33]. Furthermore, complex hydrides are generally defined as compounds with the general formula M(XHx)y, in which M and X represent metal cations and metal or non-metal elements that have covalent or iono-covalent bonding with hydrogen. Complex hydrides include alanates (e.g., LiAlH4 (10.4 wt%) and Mg(AlH4)2 (9.7 wt%)), amide–hydride composites (e.g., LiNH2-2LiH (11.5 wt%)), metal B-based complex hydrides (e.g., LiBH4(NH3BH3) (18.9 wt%), Mn(BH4)2·6NH3 (14.0 wt%)), and metalorganic hydrides [34]. Additionally, chemical hydrides are a promising option. Chemical hydrides are lighter than metal hydrides and have higher hydrogen densities. LiH (25.2 wt%), LiAlH (21.1 wt%), NaBH (21.3 wt%), and NH3BH3 (19.6 wt%) are promising chemical hydrides [35]. Although these complex and chemical hydrides have high hydrogen density, they continue to face several problems, including low reversibility, thermodynamic limitations during dehydrogenation, slow kinetics during hydrogenation and dehydrogenation, and potential evolution of another product [36].
Hydrogen can also be stored via adsorption, in which hydrogen molecules are physically bonded through van der Waals bonding with a material with a large specific surface area. However, as van der Waals bonding is relatively weak (3 kJ/mol-H2–10 kJ/mol-H2), gaseous hydrogen must be charged at relatively high pressures and low temperatures [37] to achieve a relatively high hydrogen storage density. The pressure during hydrogen charging is 1–10 MPa (depending on the adsorbent materials and application), while liquid nitrogen is generally adopted as the cooling medium [38]. Several adsorbents have been developed, including zeolites [39], metal organic frameworks (MOFs) [40], porous carbon materials [41], and porous polymeric materials [37]. Low adsorbent density, the requirement for additives to enhance heat conductivity, low volumetric hydrogen density [42], and requirement for heat management are challenges faced by hydrogen adsorption. The adsorption is exothermic; therefore, heat removal is required to facilitate a sufficient level of adsorption [37].
LOHC is a liquid that can store and release hydrogen reversibly through hydrogenation and dehydrogenation processes, respectively. The hydrogen density of LOHCs was in the range of 5–7 wt%. Promising LOHCs included toluene (C7H7)/methyl cyclohexane (C7H14), benzene (C6H6)/cyclohexane (C6H12), naphthalene (C10H8)/decalin (C10H18), biphenyl (C12H10)/bicyclohexyl (C12H22), and dibenzyltoluene (H0-DBT)/perhydrodibenzyltoluene (H18-DBT) with hydrogen storage densities of 6.2, 7.2, 7.3, 7.23, and 6.2%, respectively [43][44][45]. LOHCs are essentially liquid under atmospheric conditions (20 °C and 1 atm); therefore, their handling, storage, and transportation are highly convenient. In addition, it is stable, safe, and compatible with the existing fuel infrastructure [44]. However, LOHCs have disadvantages, such as low hydrogen density, the large amount of energy required during dehydrogenation, and the need for purification after dehydrogenation [46].
Hydrogen can also be physically stored in liquid conditions at a temperature of −253 °C. This liquefaction leads to high gravimetric and volumetric hydrogen densities of 100 wt% and 70.9 kg-H2/m3, respectively, which are higher than those of compressed hydrogen, hydrides, and adsorption-based hydrogen storage. Furthermore, the liquefaction of hydrogen leads to several possibilities of storage, including liquid hydrogen (at normal pressure), cryo-compression (at elevated pressure), and slush (suspension with solid) hydrogen. Hydrogen becomes supercritical at temperatures and pressures higher than −240 °C and 1.3 MPa, respectively. Cryo-compressed storage refers to a combination of cryogenic liquid and compressed storage [47]. This combination leads to a higher hydrogen storage density than liquid hydrogen, no change in phase, reduction in evaporation, increase in pressure buildup time, and reduction of boil-off losses [48][49]. However, the heat transferred from the surroundings results in evaporation, and the pressure inside the vessel increases accordingly. When the pressure limit was reached, the boil-off valve opened. Cryo-compressed hydrogen has several challenges, such as tank design, material, and expensive refueling infrastructure.
Slush hydrogen is defined as a cryogenic suspension of combined sub-cooled liquid and solid hydrogen at a triple point (−259.3 °C, 7.042 kPa) [50], and it has a higher gravimetric density (approximately 15–20% higher) than liquid hydrogen [51]. When the hydrogen slush contains 50% mass fraction of hydrogen solid, the gravimetric density and heat capacity are increased by 15.5 and 18.3%, respectively, compared to the liquid hydrogen at its boiling temperature. It has a higher density and heat capacity compared to liquid hydrogen, and is mainly adopted in aerospace rockets as a fuel [52]. Moreover, slush hydrogen can be achieved through any repeated freeze–thaw process, in which liquid hydrogen is brought near its boiling point and the pressure is reduced. This results in vaporization of liquid hydrogen, removing the latent heat, and decreasing its temperature [53]. When the liquid hydrogen is subsequently cooled down and its triple point is reached, solid hydrogen is formed on the surface of the vaporizing liquid. As the vacuuming is stopped, the pressure increases, leading to the melting of the formed solid hydrogen before it sinks and is agitated in liquid hydrogen. This process is repeated.
Storing hydrogen in the form of organic fuels, including methane and methanol, is considered non-carbon-free, as these materials involve carbon in their molecules. Among reformed organic fuels, ammonia is also considered promising for storing hydrogen due to its high hydrogen density (17.8 wt%), availability of infrastructure, wide possibility for utilization (with and without decomposition), and good storability (liquefaction at pressure of 0.8 MPa and temperature of 20 °C) [26][54]. However, ammonia faces several challenges, including high energy demand during its synthesis, narrow flammability range (15.15–27.35% and 15.95–26.55% in dry and 100% relative humidity of air, respectively), relatively higher apparent toxicity (approximately three orders of magnitude higher than methanol), and its potential to generate NOx during its combustion at high temperatures [52]. In addition, ammonia decomposition to release hydrogen requires a large amount of energy of 30.6 kJ/mol-H2.
Large-scale hydrogen storage demands a high density of hydrogen storage. Liquid hydrogen and ammonia are considered promising storage methods, considering their hydrogen storage density and utilization. According to Wijayanta et al. [26], liquid hydrogen is the most economically competitive when high-purity hydrogen is required during utilization. In addition, liquid hydrogen remains highly competitive compared to ammonia in many carbon-neutral applications. Liquid hydrogen is predicted to be applicable for advanced applications demanding high gravimetric energy density, such as maritime and aviation.
References
- International Energy Agency (IEA) Global CO2 Emissions in 2019. Available online: https://www.iea.org/articles/global-co2-emissions-in-2019 (accessed on 28 July 2021).
- United Nations Framework Convention on Climate Change (UNFCCC) Kyoto Protocol to the United Nations Framework Convention on Climate Change. Available online: https://unfccc.int/resource/docs/convkp/kpeng.pdf (accessed on 28 July 2021).
- United Nations Framework Convention on Climate Change (UNFCCC) Report of the Conference of the Parties on Its Twenty-First Session, Held in Paris from 30 November to 13 December 2015. Available online: https://cop23.unfccc.int/resource/docs/2015/cop21/eng/10a01.pdf (accessed on 28 July 2021).
- Mikul, H. Energy transition and the role of system integration of the energy, water and environmental systems. J. Clean. Prod. 2021, 292, 126027.
- Nam, E.; Jin, T. Mitigating carbon emissions by energy transition, energy efficiency, and electrification: Difference between regulation indicators and empirical data. J. Clean. Prod. 2021, 300, 126962.
- Li, Y.; Lan, S.; Ryberg, M.; Pérez-Ramírez, J.; Wang, X. A quantitative roadmap for China towards carbon neutrality in 2060 using methanol and ammonia as energy carriers. iScience 2021, 24, 102513.
- Mutezo, G.; Mulopo, J. A review of Africa’s transition from fossil fuels to renewable energy using circular economy principles. Renew. Sustain. Energy Rev. 2021, 137, 110609.
- Klemeš, J.J.; Van Fan, Y.; Jiang, P. The energy and environmental footprints of COVID-19 fighting measures—PPE, disinfection, supply chains. Energy 2020, 211, 118701.
- Lata, D.; Misra, A. Theoretical and experimental investigations on the performance of dual fuel diesel engine with hydrogen and LPG as secondary fuels. Int. J. Hydrogen Energy 2010, 35, 11918–11931.
- Juste, G.L. Hydrogen injection as additional fuel in gas turbine combustor. Evaluation of effects. Int. J. Hydrogen Energy 2006, 31, 2112–2121.
- Juangsa, F.B.; Prananto, L.A.; Mufrodi, Z.; Budiman, A.; Oda, T.; Aziz, M. Highly energy-efficient combination of dehydrogenation of methylcyclohexane and hydrogen-based power generation. Appl. Energy 2018, 226, 31–38.
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035.
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693.
- Yin, L.; Ju, Y. Review on the design and optimization of hydrogen liquefaction processes. Front. Energy 2020, 14, 530–544.
- Valenti, G. 2-Hydrogen liquefaction and liquid hydrogen storage. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2016; pp. 27–51. ISBN 978-1-78242-362-1.
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719.
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512.
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033.
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527.
- Najjar, Y.S. Hydrogen safety: The road toward green technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728.
- Hydrogen Tools Basic Hydrogen Properties. Available online: https://h2tools.org/hyarc/hydrogen-data/basic-hydrogen-properties (accessed on 26 June 2021).
- University of Florida Liquid Hydrogen. Available online: https://www.ehs.ufl.edu/programs/lab/cryogens/hydrogen/ (accessed on 12 May 2021).
- H2 Tools Hydrogen Density at Different Temperatures and Pressures. Available online: https://h2tools.org/hyarc/hydrogen-data/hydrogen-density-different-temperatures-and-pressures (accessed on 28 July 2021).
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657.
- Antonelli, M.; Barsali, S.; Desideri, U.; Giglioli, R.; Paganucci, F.; Pasini, G. Liquid air energy storage: Potential and challenges of hybrid power plants. Appl. Energy 2017, 194, 522–529.
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrogen Energy 2019, 44, 15026–15044.
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062.
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing Hydrogen Storage Properties of MgH2 by Transition Metals and Carbon Materials: A Brief Review. Front. Chem. 2020, 8, 552.
- Juangsa, F.B.; Irhamna, A.R.; Aziz, M. Production of ammonia as potential hydrogen carrier: Review on thermochemical and electrochemical processes. Int. J. Hydrogen Energy 2021, 46, 14455–14477.
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-pressure gaseous hydrogen permeation test method -property of polymeric materials for high-pressure hydrogen devices (1). Int. J. Hydrogen Energy 2020, 45, 29082–29094.
- Shafiee, S.; McCay, M.H. Different reactor and heat exchanger configurations for metal hydride hydrogen storage systems—A review. Int. J. Hydrogen Energy 2016, 41, 9462–9470.
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140.
- Di Profio, P.; Arca, S.; Rossi, F.; Filipponi, M. Comparison of hydrogen hydrates with existing hydrogen storage technologies: Energetic and economic evaluations. Int. J. Hydrogen Energy 2009, 34, 9173–9180.
- He, T.; Cao, H.; Chen, P. Complex Hydrides for Energy Storage, Conversion, and Utilization. Adv. Mater. 2019, 31, e1902757.
- Semelsberger, T.A. Fuels—Hydrogen Storage|Chemical Carriers. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 504–518. ISBN 978-0-444-52745-5.
- Weidenthaler, C.; Felderhoff, M. Complex Hydrides. In Handbook of Hydrogen Storage; Wiley: Hoboken, NJ, USA, 2010; pp. 117–157.
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919.
- Berenguer-Murcia, Á.; Marco-Lozar, J.P.; Cazorla-Amorós, D. Hydrogen Storage in Porous Materials: Status, Milestones, and Challenges. Chem. Rec. 2018, 18, 900–912.
- Weitkamp, J.; Fritz, M.; Ernst, S. Zeolites as Media for Hydrogen Storage. In Proceedings of the Ninth International Zeolite Conference, Montreal, QC, Canada, 5–10 July 1993.
- Langmi, H.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen Storage in Metal-Organic Frameworks: A Review. Electrochim. Acta 2014, 128, 368–392.
- Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. A 2013, 1, 9365–9381.
- García-Holley, P.; Schweitzer, B.; Islamoglu, T.; Liu, Y.; Lin, L.; Rodriguez, S.; Weston, M.H.; Hupp, J.T.; Gómez-Gualdrón, D.A.; Yildirim, T.; et al. Benchmark Study of Hydrogen Storage in Metal–Organic Frameworks under Temperature and Pressure Swing Conditions. ACS Energy Lett. 2018, 3, 748–754.
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823.
- Hurskainen, M.; Ihonen, J. Techno-economic feasibility of road transport of hydrogen using liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2020, 45, 32098–32112.
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040.
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment based on chemical and economic properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654.
- Klell, M. Storage of Hydrogen in the Pure Form. In Handbook of Hydrogen Storage; Wiley: Hoboken, NJ, USA, 2010; pp. 1–37.
- Moreno-Blanco, J.; Petitpas, G.; Espinosa-Loza, F.; Elizalde-Blancas, F.; Martinez-Frias, J.; Aceves, S.M. The storage performance of automotive cryo-compressed hydrogen vessels. Int. J. Hydrogen Energy 2019, 44, 16841–16851.
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840.
- Gürsu, S.; Sheriff, S.A.; Vezirocğlu, T.N.; Sheffield, J.W. Review of slush hydrogen production and utilization technologies. Int. J. Hydrogen Energy 1994, 19, 491–496.
- Ohira, K. 3-Slush hydrogen production, storage, and transportation. In Compendium of Hydrogen Energy; Gupta, R.B., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2016; pp. 53–90. ISBN 978-1-78242-362-1.
- Jin, T.; Li, Y.; Liang, Z.; Lan, Y.; Lei, G.; Gao, X. Numerical prediction of flow characteristics of slush hydrogen in a horizontal pipe. Int. J. Hydrogen Energy 2017, 42, 3778–3789.
- Park, Y.M. Literature research on the production, loading, flow, and heat transfer of slush hydrogen. Int. J. Hydrogen Energy 2010, 35, 12993–13003.
- Juangsa, F.B.; Aziz, M. Integrated system of thermochemical cycle of ammonia, nitrogen production, and power generation. Int. J. Hydrogen Energy 2019, 44, 17525–17534.
More
Information
Subjects:
Engineering, Environmental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.9K
Revisions:
2 times
(View History)
Update Date:
27 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




