
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karolina Walczyńska-Dragon | + 1922 word(s) | 1922 | 2021-09-13 05:57:15 |
Video Upload Options
Dental plaque is a community of microorganisms found on the surface of teeth or other hard surfaces like dentures and embedded in a matrix of polymers of both host and bacterial origin.
1. Introduction
The microorganisms have the ability to attach to other microorganisms in a way that allows them to survive and resist host defense mechanisms or antibiotic treatments. Most of them attach to different surfaces to form some type of biofilm matrix that is highly structured and spatially organized.
If not removed regularly, the biofilm undergoes maturation, which is connected with a progressive shift from a Gram-positive to a Gram-negative anaerobic species, which results in formation under the gingival surface, where bacteria grow profusely [1]. The pathogenic bacterial complex can lead to dental caries, periodontitis and gingivitis. Tissue injury, flossing, dental treatment and even chewing and eating can induce blood vessel injury near spaces covered in dental plaque. It allows the leakage of Gram-negative anaerobic species into the systemic bloodstream followed by bacteremia.
In addition, dental biofilm, especially subgingival plaque in patients with periodontitis, has been associated with cardiovascular and respiratory disease and diabetes mellitus, and recently constituting a high risk for developing severe illness due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection [2].
The Role of Dental Plaque in The Etiology of Dental Caries and Periodontitis
It was shown that 40–50% of plaque remains after tooth brushing, and the effects of biofilm retention are clearly evident in the prevalence of caries, gingivitis and periodontal disease. Sucrose fermentation causes pH to drop rapidly to 5.0 or less at the point where the plaque contacts the tooth enamel. The enzyme invertase splits sucrose into its component glucose and fructose molecules, which are then converted to lactic acid by the glycolytic pathway [3].
When the pH of the dental plaque drops below 5.0–5.2, the saliva buffer cannot compensate. Tooth mineral is solubilized, thereby buffering the plaque and maintaining an environment suitable for Streptococcus mutans growth. Then the lactic acid that comes from the bacterial fermentation of sucrose and other carbohydrates in the diet reach the enamel surface, which begins to dissolve, releasing Ca and PO 4 ions from the surfaces under the enamel [4].
Presence of plaque rich in bacteria (especially Streptococcus mutans ) directly destroys the enamel layer by dissolving tooth minerals (mainly hydroxyapatite, Ca 10(PO 4) 6(OH) 2) resulting in caries. Periodontal diseases include two main conditions: gingivitis and periodontitis. Periodontitis is a complex, chronic inflammatory disease caused by an abnormal host response to bacteria from dental plaque. It affects the supporting structures of the teeth (root cementum, periodontal ligament and alveolar bone), causing irreversible attachment and bone loss, which are observed histologically and clinically. It leads finally to tooth loss. It has been estimated that periodontitis affects about 40% of the adult population. The transition from a healthy periodontium to a diseased one depends on the microbial dysbiosis and the abnormal immune host response. While the more severe forms of periodontal disease associated with alveolar bone loss are less frequent, gingivitis is widespread at all ages and is the most common form of periodontal disease.
It was shown, that in patients with severe periodontitis, there was a significant reduction in serum albumin concentration and an increase in loss of attachment [5].
2. Possible Influence of Dental Plaque on Severe COVID-19 Complications
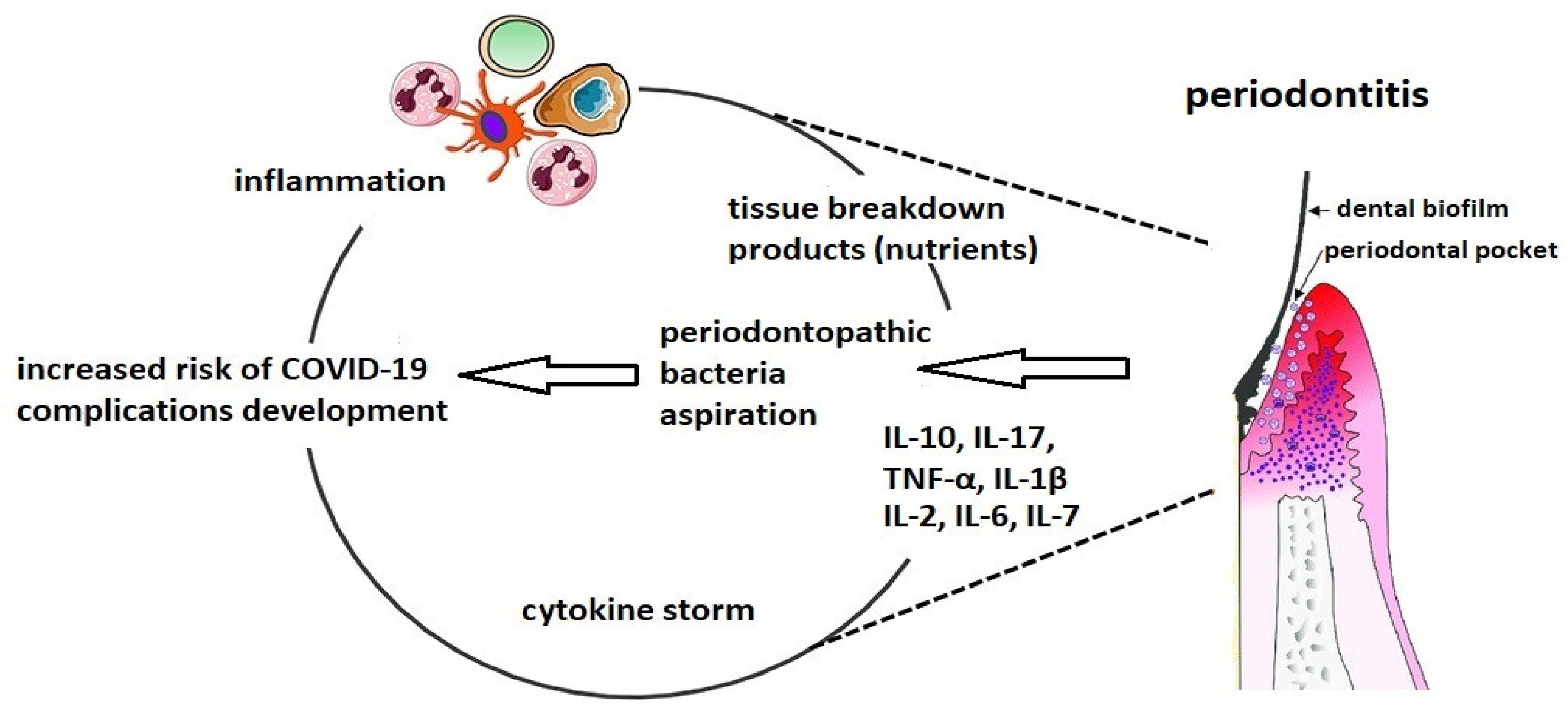
Figure 1. The connection between altered oral biofilm and complications of COVID-19.
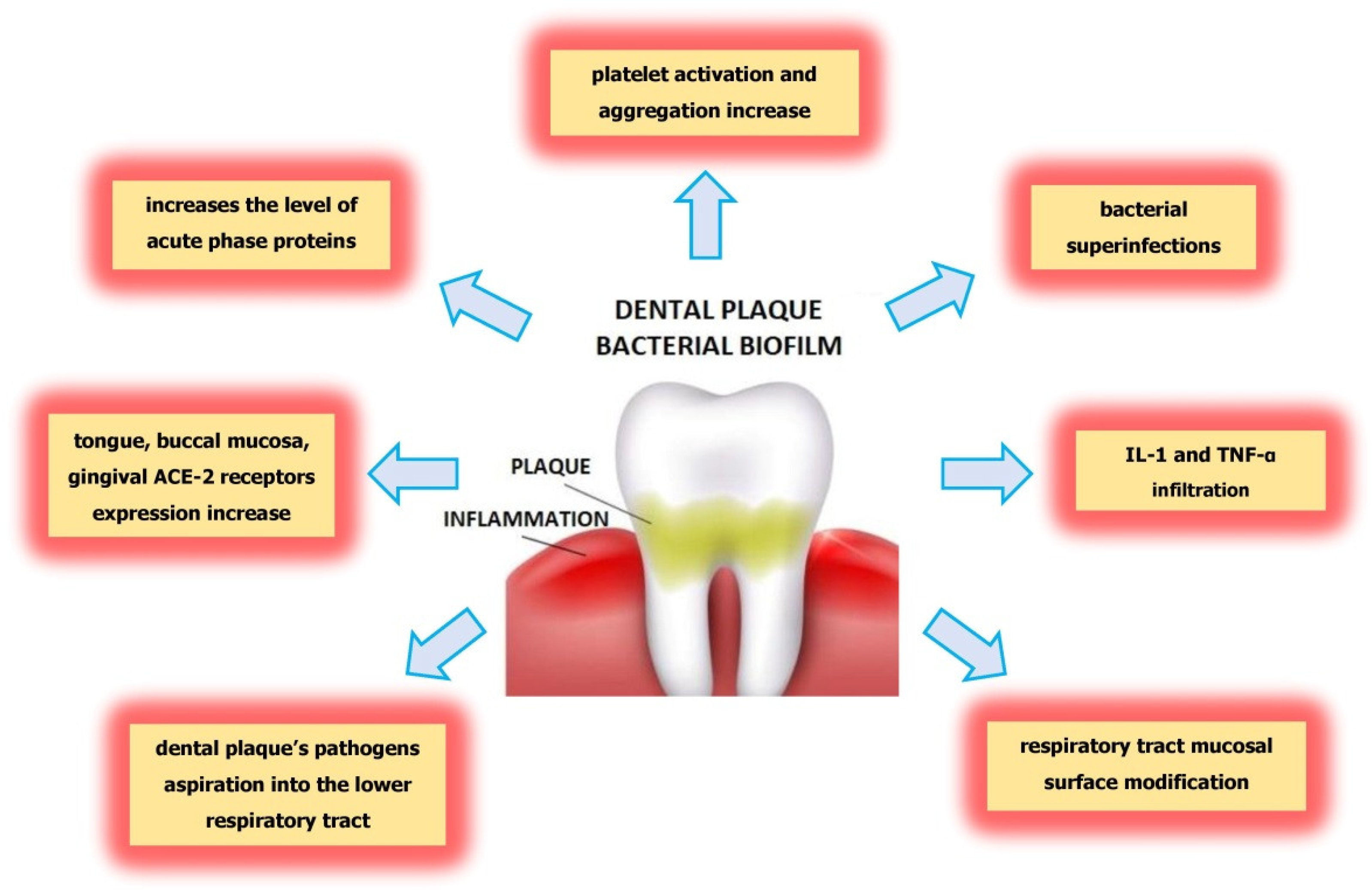
3. Conclusions
References
- Yumoto, H.; Hirota, K.; Hirao, K.; Ninomiya, M.; Murakami, K.; Fujii, H.; Miyake, Y. The Pathogenic Factors from Oral Streptococci for Systemic Diseases. Int. J. Mol. Sci. 2019, 20, 4571.
- Kleinstein, S.; Nelson, K.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139.
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86.
- Dunne, W.M. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166.
- Kaur, N.; Kaur, N.; Sarangal, V. A study to evaluate the correlation of serum albumin levels with chronic periodontitis. Ind. J. Dent. Res. 2015, 26, 11–14.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059.
- Knypl, K. Pharmacotherapy of arterial hypertension: Antagonists of angiotensin receptors AT1. Med. Rodz. 2001, 2, 58–60.
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8.
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Kobayashi, R.; Iinuma, T.; Imai, K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J. Oral Sci. 2021, 63, 1–3.
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Yokoe, S.; Suzuki, R.; Sato, S.; Iinuma, T.; Imai, K. Expression of the SARS-CoV-2 Receptor ACE2 and Proinflammatory Cytokines Induced by the Periodontopathic Bacterium Fusobacterium nucleatum in Human Respiratory Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1352.
- Gupta, S.; Mohindra, R.; Chauhan, P.; Singla, V.; Goyal, K.; Sahni, V.; Gaur, R.; Verma, D.; Ghosh, A.; Soni, R.; et al. SARS-CoV-2 Detection in Gingival Crevicular Fluid. J. Dent. Res. 2021, 100, 187–193.
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 1–7.
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and periodontal disease. J. Int. Med Res. 2021, 49, 3000605211002695.
- Praczyk, Ł.; Hoffmann, K.; Bryl, W. Galectin 3 as a Biomarker in Cardiovascular Diseases. HYGIEA Public Health 2019, 54, 75–79. Available online: http://www.h-ph.pl/pdf/hyg-2019/hyg-2019-2-075.pdf (accessed on 4 March 2021).
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yücel, Ç.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and PGE2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, JJID.2021.020.
- Kara, C.; Çelen, K.; Dede, F.O.; Gökmenoğlu, C.; Kara, N.B. Is periodontal disease a risk factor for developing severe Covid-19 infection? The potential role of Galectin-3. Exp. Biol. Med. 2020, 245, 1425–1427.
- Liu, F.-T. Galectins: A New Family of Regulators of Inflammation. Clin. Immunol. 2000, 97, 79–88.
- De Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and auto-immune diseases. Exp. Biol. Med. 2015, 240, 1019–1028.
- Argüeso, P.; Panjwani, N. Focus on Molecules: Galectin-3. Exp. Eye Res. 2011, 92, 2–3.
- Cao, W.; Li, T. COVID-19: Towards understanding of pathogenesis. Cell Res. 2020, 30, 367–369.
- Herzberg, M.C.; Meyer, M.W. Effects of Oral Flora on Platelets: Possible Consequences in Cardiovascular Disease. J. Periodontol. 1996, 67, 1138–1142.
- Dikshit, S. Fibrinogen Degradation Products and Periodontitis: Deciphering the Connection. J. Clin. Diagn. Res. 2015, 9, ZC10–ZC12.
- Almeida-da-Silva, C.L.C.; Alpagot, T.; Zhu, Y.; Lee, S.S.; Roberts, B.P.; Hung, S.-C.; Tang, N.; Ojcius, D.M. Chlamydia pneu-moniae is present in the dental plaque of periodontitis patients and stimulates an inflammatory response in gingival epithelial cells. Microb. Cell 2019, 6, 197–208.
- Gomes-Filho, I.; Passos-Soares, J.; Da Cruz, S.S. Respiratory disease and the role of oral bacteria. J. Oral Microbiol. 2010, 2, 5811.
- Varanat, M.; Haase, E.; Kay, J.; Scannapieco, F. Activation of the TREM-1 pathway in human monocytes by periodontal pathogens and oral commensal bacteria. Mol. Oral Microbiol. 2017, 32, 275–287.
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975.
- Paju, S.; A Scannapieco, F. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007, 13, 508–512.
- Wojtkowska, A.A.; Wysokiński, A. Periodontitis and prevalence of cardiovascular diseases. Chor. Serca I Naczyń 2015, 12, 289–294.
- Botros, N.; Iyer, P.; Ojcius, D.M. Is there an association between oral health and severity of COVID-19 complications? Biomed. J. 2020, 43, 325–327.
- Yang, L.-C.; Suen, Y.-J.; Wang, Y.-H.; Lin, T.-C.; Yu, H.-C.; Chang, Y.-C. The Association of Periodontal Treatment and Decreased Pneumonia: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 356.




