
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andres Escobar | + 2522 word(s) | 2522 | 2021-09-13 09:36:32 | | | |
| 2 | Vicky Zhou | Meta information modification | 2522 | 2021-09-24 02:53:02 | | |
Video Upload Options
Integrated microfluidic platforms (IMPs) refer to diagnostic or analytic platforms that utilize, in part, a microfluidic-based system to facilitate the combination of several different steps of medical diagnoses toward full or partial automation. The integration of microfluidics in novel diagnostic technologies takes advantage of micrometric-scale fluids behaving very differently than fluids at volumes seen in our everyday lives. The two main types of currently existing microfluidic systems are continuous-flow and droplet-based systems that can both be further classified based on their respective materials and detection methods.
1. Introduction
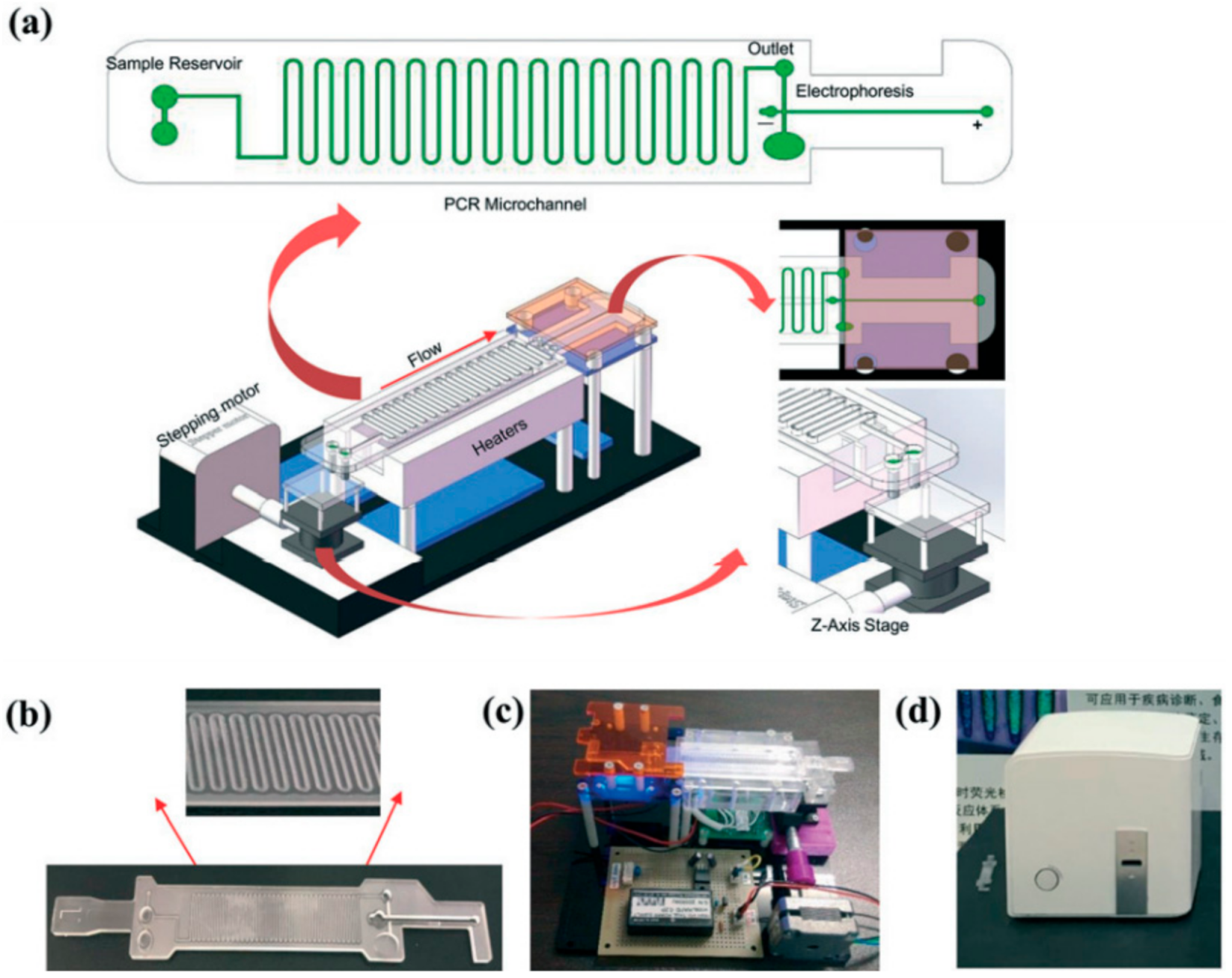
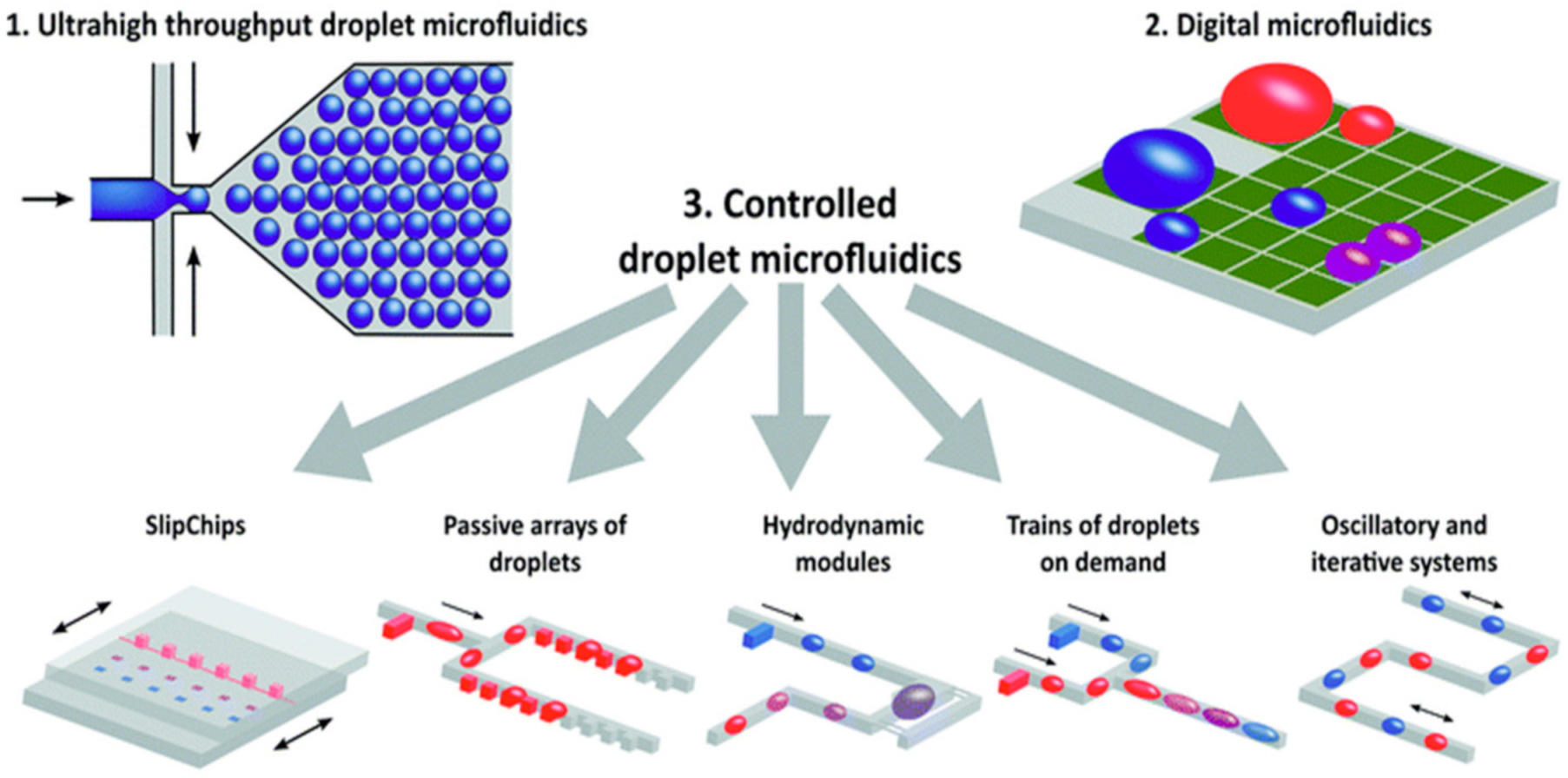
2. Clinical Translation of Integrated Microfluidic Devices for Detection and Quantification of SARS-CoV-2
Current microfluidic devices for the detection and quantification of SARS-CoV-2 in patient samples hold great promise for future on-site diagnostic use. The increase in detection speed, specificity and sensitivity achieved through microfluidic integration largely improve the potential use of these devices in low-resource settings where access to diagnostic tests is severely limited [8][9]. However, many of these techniques are limited by the cost of fabrication in mass production, as well as in their robustness and durability outside of laboratory settings. The hope is that with modifications, these different devices and techniques might be able to eventually achieve microfluidic integration that can be used on-site and reliably diagnose patients infected with SARS-CoV-2.
In Table 1 , we present several notable characteristics of four of the most promising SARS-CoV-2 detection technologies currently available. The listed technologies have shown significant advancements in their potential for clinical translation through the integration of microfluidics. One example of note is the work of Qu et al. on microflow cytometry [10]. Despite not currently existing on a mobile platform, the low detection limit, extremely short total assay time and the relatively low cost of this technology demonstrates the effectiveness of novel microfluidic-based integrated diagnostic devices for detecting SARS-CoV-2 in extremely small sample volumes [10]. Although many of the conventional detection methods, such as immunoassay and RT-PCR, have comparable specificity, sensitivity and short turnaround time as a result of microfluidic integration, it does not compare well to other more novel methods. Detection methods such as microflow cytometry and nanoparticle-based detection may offer more of an inherent advantage to SARS-CoV-2 diagnosis solely based on factors such as detection limit, sample volume and quantitative capabilities; however, as previously mentioned, an optimal on-site integrated microfluidic diagnostic device for SARS-CoV-2 detection must adhere to the ASSURED standards set out by the WHO and the seven pillars of microfluidic integration to the best of their ability [11]. Therefore, microfluidic integration appears to hold the most promise in facilitating the clinical translation of our currently existing technology toward a cost-effective, rapid and selective diagnostic tool for SARS -CoV-2 that will be more readily accessible to people from low-resource areas, as well as other parts of the world.
| Immunoassay | RT-PCR | Nanoparticle | Microflow Cytometry |
|
|---|---|---|---|---|
| Reagent Consumption |
10 µg (in tube) | 20 µL (in tube) | Negligible | 50 µL (in tube) |
| Target of Detection | IgG, IgA, IgM | N gene, E gene | Gold-spiked | IgM, IgG |
| Limit of Detection | 0.15 mg/L | 1-10 copy per µL | 0.08 mg/L | 0.06-0.10 mg/L |
| Total Assay Time | 1 h | 2 h | 2–5 h | 30 min |
| Sample Volume | 20 µL | 120 µL | 1 µL | 10 µL |
| Assay Control | Automated | Manual | Manual | Automated |
| Cost per Test | ~ 6 (USD) | ~ 4 (USD) | ~ 10 (USD) | ~ 5 (USD) |
| Quantitative | No | Yes | Yes | Yes |
| Mobile | Yes | Yes | No | No |
Stage-Wise Implementation of Microfluidic SARS-CoV-2 Detection
3. Conclusions
References
- Kaminski, T.S.; Garstecki, P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem. Soc. Rev. 2017, 46, 6210–6226.
- Tarim, E.A.; Karakuzu, B.; Oksuz, C.; Sarigil, O.; Kizilkaya, M.; Al-Ruweidi, M.K.A.A.; Yalcin, H.C.; Ozcivici, E.; Tekin, H.C. Microfluidic-based virus detection methods for respiratory diseases. Emergent Mater. 2021, 4, 143–168.
- Garneret, P.; Coz, E.; Martin, E.; Manuguerra, J.; Brient-Litzler, E.; Enouf, V.; Obando, D.F.G.; Olivo-Marin, J.; Monti, F.; van der Werf, S.; et al. Performing point-of-care molecular testing for SARS-CoV-2 with RNA extraction and isothermal amplification. PLoS ONE 2021, 16, e0243712.
- Li, Z.; Ju, R.; Sekine, S.; Zhang, D.; Zhuang, S.; Yamaguchi, Y. All-in-one microfluidic device for on-site diagnosis of pathogens based on an integrated continuous flow PCR and electrophoresis biochip. Lab Chip 2019, 19, 2663–2668.
- Kulkarni, M.B.; Goel, S. Advances in continuous-flow based microfluidic PCR-devices—A review. Eng. Res. Express 2020, 2, 042001.
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60.
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Accounts Chem. Res. 2013, 46, 2396–2406.
- Khan, M.S.; Tariq, M.O.; Nawaz, M.; Ahmed, J. MEMS Sensors for Diagnostics and Treatment in the Fight Against COVID-19 and Other Pandemics. IEEE Access 2021, 9, 61123–61149.
- Funari, R.; Chu, K.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578.
- Qu, J.; Chenier, M.; Zhang, Y.; Xu, C. A Microflow Cytometry-Based Agglutination Immunoassay for Point-of-Care Quantitative Detection of SARS-CoV-2 IgM and IgG. Micromachines 2021, 12, 443.
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid Tests for Sexually Transmitted Infections (STIs): The Way Forward. Sex. Transm. Infect. 2006, 82, v1–v6.
- Swank, Z.; Michielin, G.; Yip, H.M.; Cohen, P.; Andrey, D.O.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; Meyer, B.; Maerkl, S.J. A High-Throughput Microfluidic Nanoimmunoassay for Detecting Anti–SARS-CoV-2 Antibodies in Serum or Ultralow-Volume Blood Samples. Proc. Natl. Acad. Sci. USA 2021, 118, e2025289118.
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110.
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057.
- WHO. COVID-19 Weekly Epidemiological Update: 23 August 2021; World Health Organization: Geneva, Switzerland, 2021; Volume 41, pp. 1–31.
- Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel 2021; Division of Viral Diseases CDC-006-00019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus (COVID-19) Testing. In Our World Data; Oxford Martin School, Oxford University: Oxford, UK, 2021; Available online: https://ourworldindata.org/coronavirus-testing (accessed on 23 August 2021).
- Abbott Receives CE Mark for Its PanbioTM. Rapid Antigen Self-Test, Opening Access Throughout Europe to Fast, Reliable COVID-19 Testing. Available online: https://abbott.mediaroom.com/2021-06-28-Abbott-Receives-CE-Mark-for-its-Panbio-TM-Rapid-Antigen-Self-Test,-Opening-Access-Throughout-Europe-to-Fast,-Reliable-COVID-19-Testing (accessed on 21 August 2021).
- Kaarj, K.; Akarapipad, P.; Yoon, J.-Y. Simpler, Faster, and Sensitive Zika Virus Assay Using Smartphone Detection of Loop-mediated Isothermal Amplification on Paper Microfluidic Chips. Sci. Rep. 2018, 8, 12438.
- Yeh, E.-C.; Fu, C.-C.; Hu, L.; Thakur, R.; Feng, J.; Lee, L.P. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci. Adv. 2017, 3, e1501645.
- Kawasuji, H.; Takegoshi, Y.; Kaneda, M.; Ueno, A.; Miyajima, Y.; Kawago, K.; Fukui, Y.; Yoshida, Y.; Kimura, M.; Yamada, H.; et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS ONE 2020, 15, e0243597.
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020, 11, 5493.
- FDA. Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers; FDA: Silver Spring, MD, USA, 2021.





