
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junsha An | + 2663 word(s) | 2663 | 2021-09-17 10:10:16 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2663 | 2021-09-18 08:26:15 | | |
Video Upload Options
Breast cancer has an extremely high incidence in women, and its morbidity and mortality rank first among female tumors. With the increasing development of medicine today, the clinical application of neoadjuvant chemotherapy has brought new hope to the treatment of breast cancer. Based on the relevant research on the existing drug resistance mechanism, the current treatment plan for reversing the resistance of breast cancer to neoadjuvant chemotherapy is explored, and the potential drug targets are analyzed, aiming to provide a new idea and strategy to reverse the resistance of neoadjuvant chemotherapy drugs in breast cancer.
1. Background
Breast cancer is currently a cancer with an extremely high incidence in women, and its mortality rank first among female tumors [1]. According to the data of GLOBOCAN in 2018, about 2.1 million patients are diagnosed with breast cancer, and the death toll is 630,000 [2]. Today, with changes in the environment and lifestyle, the incidence of breast cancer is also increasing [3]. New statistics show that breast cancer still ranks first among female cancers. Therefore, overcoming breast cancer has increasingly become a problem of global concern.
In clinical treatment of breast cancer, surgery is usually combined with chemotherapy. With the development of biology and immunology, the approach to breast cancer treatment is constantly updated. In recent years, breast cancer has been considered a systemic disease, and neoadjuvant chemotherapy has also been included as an important part of the treatment of breast cancer.
Neoadjuvant chemotherapy refers to systemic chemotherapy before the implementation of local treatment methods (such as surgery or radiotherapy). It is mainly suitable for patients with mid-stage and locally advanced breast cancer. The concept of neoadjuvant chemotherapy was formally proposed by Rosen et al. in 1979. It aims to transform inoperable breast cancer into operable breast cancer, convert breast cancer that requires breast removal into breast-sparing breast cancer and provide drug basis in the follow-up treatment to improve the prognosis of patients [4].
At present, there is no uniform standard for neoadjuvant chemotherapy for breast cancer. In the early stage, a unified neoadjuvant chemotherapy regimen is generally given to all patients, but today’s neoadjuvant chemotherapy regimens tend to be personalized, as shown in Figure 1, generally based on curative effect prediction markers and molecular subtypes to give personalized treatment.
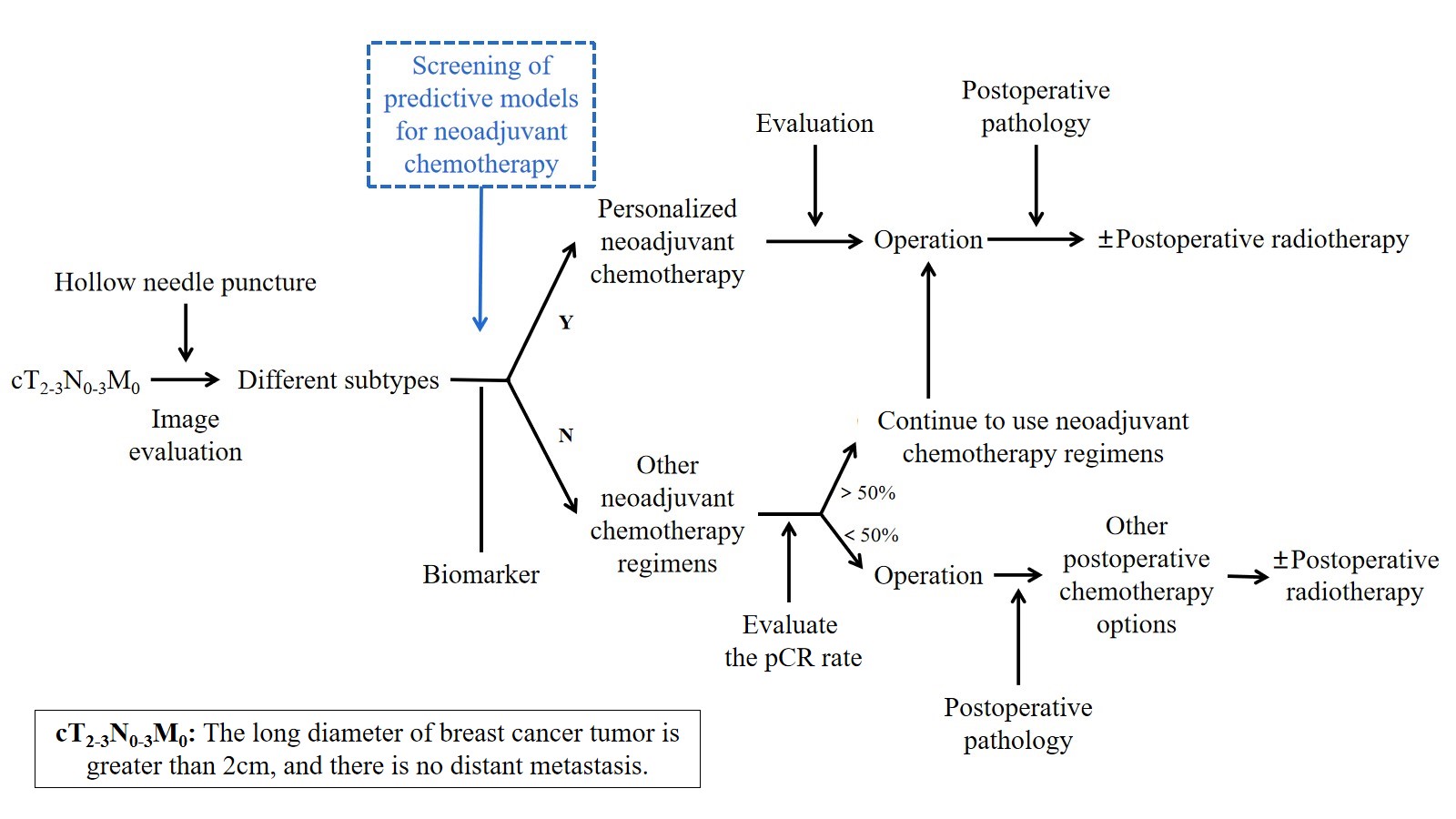
Figure 1. Personalized neoadjuvant chemotherapy.
In the use of chemotherapy drugs, anthracyclines, such as doxorubicin and epirubicin, are generally used in combination with drugs such as cyclophosphamide, epirubicin, and fluorouracil. The emergence of taxanes and their significant anti-tumor activity against advanced breast cancer have further improved the efficacy of neoadjuvant chemotherapy. In clinical use, no matter single drug or combination drugs, taxane drugs all show good anti-tumor activity. For breast cancer subtypes with HER2 overexpression, trastuzumab is generally added to the neoadjuvant chemotherapy regimen, and satisfactory results have been obtained in clinical use [5].
Although the efficacy of neoadjuvant chemotherapy has been confirmed, clinical trial data show that the effects of neoadjuvant chemotherapy for different breast cancer patients are very different, and it is easy to develop drug resistance, which is not conducive to subsequent treatment [6]. Drug resistance to neoadjuvant chemotherapy is one of the main reasons for its treatment failure, and it is one of the most challenging problems in the treatment of breast cancer today.
2. Solutions to the Reversal of Resistanceto Neoadjuvant Chemotherapy Drugs in Breast Cancer
Neoadjuvant chemotherapy drug resistance is an important issue in clinical breast cancer treatment. At present, many studies are combining new drugs or new therapies with traditional neoadjuvant chemotherapy drugs, which can significantly reverse drug resistance caused by neoadjuvant chemotherapy drugs alone. The following chapter describes some drugs that have the potential to reverse neoadjuvant chemotherapy resistance. Although these drugs have not yet been clinically applied in breast cancer, it is believed that with research advances, these novel regimens can play an important role in the treatment of breast cancer.
2.1. Combined Use of Chemotherapeutic Drugs
According to the existing research on drug resistance mechanisms, many treatment options have brought hope to reverse the resistance of breast cancer to neoadjuvant chemotherapy. One of the more commonly used methods to reverse drug resistance is the combined use of multiple chemotherapy drugs.
On the one hand, the combined use of multiple drugs that work through different molecular mechanisms can greatly improve the problem of drug resistance caused by alterations in a single mechanism, thereby ensuring the efficacy of the drug. For example, in the clinical application of neoadjuvant chemotherapy drugs, anthracyclines that act on DNA and taxanes that act on proteins are used in combination, and drugs such as cyclophosphamide and epirubicin are used at the same time. These drugs act on different targets and can greatly improve the problem of drug resistance caused by a single mechanism, thereby ensuring the efficacy of the drug. Among neoadjuvant chemotherapy drugs for breast cancer, both trastuzumab and pertuzumab specifically act on the HER2 target, and their combined application can significantly enhance the sensitivity of breast cancer cells to neoadjuvant chemotherapy drugs. However, this method could inevitably enhance drug toxicity. In clinical treatment, it is also necessary to pay attention to the interaction between different drugs, detect the plasma drug concentration in time, and change the dose to obtain the best therapeutic effect.
On the other hand, while using neoadjuvant chemotherapy drugs, increasing the use of drugs that can block the drug resistance mechanism of tumors can restore the sensitivity of drug-resistant cells to drugs and enhance the efficacy of drugs. Most of these drugs are P-gp inhibitors, but due to high toxicity and pharmacokinetic effects, they have not been used in clinical practice [7]. The current research direction is to develop more efficient and low-toxic chemical drug reversal agents. Dual specificity phosphatase6 (DUSP6) inhibitors and histone deacetylase inhibitors (HDACI) have been identified as agents with the potential to reverse tumor drug resistance warranting follow-up research associated with drug development [8][9].
2.2. Chinese Medicine Reversal Agents
With the modern development of Chinese medicine, herbs and extractions from traditional Chinese medicine as drugs for treating tumors have gradually demonstrated their unique curative effects in the clinic [10][11][12][13]. More and more studies have shown that traditional Chinese medicine plays an important role in the prevention and treatment of breast cancer, contributing to the reversal of drug resistance of breast cancer. Moreover, compared with the above-mentioned chemical drug reversal agents, traditional Chinese medicine has the characteristics of safer, multi-component, multi-stage, and multi-targeted action. This makes traditional Chinese medicine monomers and extracts gradually attract more researchers’ attention as tumor drug reversal agents.
Many traditional Chinese medicine preparations have been developed and used in clinical practice, such as Elemene Injection and Shenqi Fuzheng Injection. The main active ingredient of Elemene Injection is a mixture of β-, γ-, and σ-elemene, which is an anti-cancer active ingredient extracted from Curcuma wenyujin Y.H.Chen. The combination of Elemene Injection and neoadjuvant chemotherapy drugs can reverse drug resistance of breast cancer cells, and its mechanism of action is mainly to inhibit the expression of P-gp, and reverse drug resistance of breast cancer cells through exosome and EMT inhibition [14][15][16][17]. Shenqi Fuzheng Injection is an injection made with Codonopsis and Astragalus as the main components. As early as 1999, Shenqi Fuzheng Injection has been formally approved by CFDA for adjuvant anti-tumor therapy [18]. Regarding its mechanism of reversing tumor drug resistance, the main mechanism is to induce cell cycle arrest and promote cell apoptosis [19]. However, it has not been used in neoadjuvant chemotherapy for breast cancer, and its mechanism of action should be further explored in the hope that it can be used in clinical practice in the future.
In addition to the above-mentioned traditional Chinese medicine compound prescriptions, there are many monomer drugs extracted from traditional Chinese medicines that have been proven to have the effect of reversing tumor resistance. Honokiol and magnolol, which are extracted from the plant Magnolia officinalis Rehd. et Wils., have a variety of pharmacological effects, such as inducing long-lasting central muscle relaxation and central nervous system inhibition, as well as anti-inflammatory, antibacterial, anti-ulcer, anti-tumor etc. Hyo-Kyung Han et al. [20] evaluated the inhibitory effects of honokiol and magnolol on P-pg activity, and found that honokiol can inhibit P-gp activity through a competitive mechanism, and both compounds can inhibit the expression of P-gp and help to improve the resistance of breast cancer to neoadjuvant chemotherapy drugs. Curcumin is derived from Curcuma longa L. In related studies, the combined treatment of curcumin and the neoadjuvant drug doxorubicin used in breast cancer can reduce the excessive efflux of doxorubicin caused by the overexpression of ABCB4, thereby enhancing the efficacy of doxorubicin [21]. Saikosaponin D is derived from Bupleurum chinense DC. It has been shown to have anti-inflammatory, antibacterial and anti-tumor effects. Relevant studies have shown that Saikosaponin D can enhance the sensitivity of breast cancer multidrug resistant cells MCF-7/ADR cells to chemotherapeutic drugs by reducing the expression of MDR1 and P-pg, which may effectively reverse the effects of neoadjuvant chemotherapy drug resistance [22]. Berberine is an alkaloid, extracted and purified from Coptis chinensis Franch. It has a variety of pharmacological effects, such as antibacterial, antihypertensive, antiarrhythmic and antitumor [23]. For reversing the drug resistance of breast cancer cells, different doses of berberine mediate different mechanisms. When using a low dose (5 µM) ofberberine, the AMPK/HIF-1α signaling pathway is inhibited and the expression of P-gp decreases, thereby increasing the sensitivity of breast cancer cells to chemotherapy drugs. The high dose (40 µM) of berberine can regulate the AMPK/HIF-1α signaling pathway, which in turn activates the expression of p53 and directly induces breast cancer cell apoptosis [24]. Resistance to paclitaxel drugs is a major obstacle in neoadjuvant chemotherapy for breast cancer, and studies have shown that gambogic acid can enhance the sensitivity of breast cancer cells to paclitaxel [25]. Gambogic acid is derived from Garcinia hanburyi Hook. f., it has anti-proliferative effects in triple-negative breast cancer cells [26]. It can also induce breast cancer cell apoptosis by inhibiting SHH signaling pathway, and has potential to be used as a combination drug in the neoadjuvant treatment of breast cancer. Ligustrazine is derived from the root of Ligusticum chuanxiong Hort. Various studies have shown that ligustrazine can block the G0/G1 phase of the cell cycle, thereby inhibiting DNA synthesis, and inducing breast cancer cell apoptosis, thereby reversing breast cancer cell resistance to neoadjuvant chemotherapy drugs [27].
Due to the complex composition of traditional Chinese medicine agents, numerous targets, and unclear mechanism of action, its follow-up research should also conduct efficacy and safety evaluations to accelerate the transformation to the clinic.
2.3. Gene Modifications
In recent years, the development of genetic engineering technology has made it possible to reverse the resistance of neoadjuvant chemotherapy drugs at the genetic level. Among them, nucleic acid-based technologies, such as siRNA, antisense oligonucleotide (ASO), mRNA, etc., have shown great potential in regulating the expression of tumor-related genes [28].
Mutations in the MEN1 gene encoding menin can cause tumors in multiple endocrine organs. For example, the occurrence and development of breast cancer are closely related to menin. Current studies have shown that inhibitors of the menin/MLL1 complex are effective in some cancers, but they are not effective in the treatment of breast cancer [29]. Recent studies have found that ASO targeting menin mRNA has a greater advantage than siRNA, and has a better curative effect in the treatment of triple-negative breast cancer [30]. More importantly, in vitro menin silencing can have a synergistic effect with the taxane drug docetaxel in neoadjuvant chemotherapy. Moreover, menin-ASO can be used in combination with neoadjuvant chemotherapy drugs that induce DNA damage or PARP inhibitors that inhibit DNA damage. It can promote cell apoptosis, which provides new ideas for neoadjuvant chemotherapy for breast cancer.
The upregulation of some pro-apoptotic genes, such as p53, can also reverse resistance of neoadjuvant chemotherapy drugs. In breast cancer cells, the tumor suppressor gene p53 is often mutated to cause abnormal expression, thereby activating the promoter of the MDR1 gene and increasing its expression. At present, the p53 gene using adenovirus as a vector, and the antisense gene of the mutant p53 gene can be used to introduce the wild-type p53 gene can be potentially used to reverse the resistance to neoadjuvant chemotherapy drugs in breast cancer [31][32].
MicroRNA (miRNA) is an endogenous 18–23-nucleotide small noncoding RNA, which can bind to the 3′-untranslated region (3’-UTR) of specific target messenger RNAs (mRNAs). Abnormal expression of miRNA, such as the oncomiRNA, microRNA-21, is overexpressed in breast cancer, which may lead to the occurrence of breast cancer, thus the regulation of miRNA is also a potential target for the treatment of breast cancer [33]. Anti-microRNA oligonucleotides (AMOs) are synthetic oligonucleotides that can complementally bind to the corresponding target miRNA, thereby inhibiting the expression of miRNA [34]. At present, most AMOs are designed for high-expressed miRNAs in breast cancer cells. Recent studies have shown that inhibiting low-expressed miRNAs in breast cancer cells, such as mir-148a of the mir-148/152 family, can also effectively inhibit the proliferation of breast cancer cells to treat breast cancer [35]. These studies show that the combination of AMO for the suppression of related miRNA expression and neoadjuvant chemotherapy has great potential to enhance the efficacy of neoadjuvant chemotherapy drugs and bring new hope for the treatment of breast cancer.
Genetic engineering mainly blocks the expression of drug-resistant genes, or acts synergistically with neoadjuvant chemotherapy drugs to promote breast cancer cell apoptosis. The development and clinical application of genetic engineering technology can effectively reverse the resistance of neoadjuvant chemotherapy drugs and improve the effect of clinical treatment of breast cancer. However, the drug resistance mechanism of breast cancer is complex, including multiple pathways and factors. For different mechanisms, the research direction should be focused on more approaches and more targets to achieve more therapeutic effects.
2.4. Immune Regulation
The current use of the immune system to reverse resistance to neoadjuvant chemotherapy drugs is mainly associated with the application of antibodies and the application of many cytokines in the body’s own immune system.
Antibodies include mainly P-gp antibodies and APO-1 monoclonal antibodies. P-gp antibodies can recognize the epitope of the P-gp membrane, competitively inhibit the pumping function of P-gp, reduce neoadjuvant chemotherapy drug efflux, enable drug accumulation in the cell, and reverse resistance to neoadjuvant chemotherapy drugs [36]. In addition, antibodies can mediate immune responses and participate in the reversal of drug resistance. The APO-1 monoclonal antibody is an antibody against the glycoprotein FAS on the cell membrane surface, which can bind to APO-1 on the breast cancer cell membrane surface to induce cell apoptosis [37]. For some breast cancer cells with low FAS expression, the FAS antigen expression vector can also be transfected into cells to promote high expression before using APO-1 monoclonal antibodies.
The cytokines of the body’s immune system, such as TNF-α, INF-α and IL-2, can reduce the expression levels of MDR1 gene mRNA and P-gp, increase the sensitivity of breast cancer cells to neoadjuvant chemotherapy drugs, and reverse resistance to neoadjuvant chemotherapy drugs [38].
2.5. Changing the Tumor Microenvironment
TME and tumor cells interact with each other. Tumor cells can create a suitable living environment for themselves by changing TME, and TME in turn regulates tumor cells, including promoting drug resistance. At present, TME is a hot spot in tumor research. It can provide new ideas for tumor treatment and reverse tumor drug resistance to a certain extent [39].
In addition to the above-mentioned HIF inhibitors and targeted inhibitors developed for the TME hypoxic environment and acidic environment, current research focus is the use of nanomaterials when analyzing TME models, and the focuses on the mechanism of nanomedicine targeting CAFs [40].
Moreover, the combination of anti-angiogenic therapy and immunotherapy can lead to a reversal of the immunospressive TME [41]. In breast cancer, anti-angiogenic therapy can induce the normalization of tumor blood vessels, and promote the recruitment of immune cells and the maturation of dendritic cells (DC) [42]. The use of immune checkpoint inhibitors can further alleviate the immunosuppressive state and promote the normalization of TME, thereby reversing resistance of neoadjuvant chemotherapy drugs induced by TME.
More importantly, the heterogeneity of TME is related to breast cancer subtypes, and the treatment plan for TME will help realize the potential of “precision medicine.”
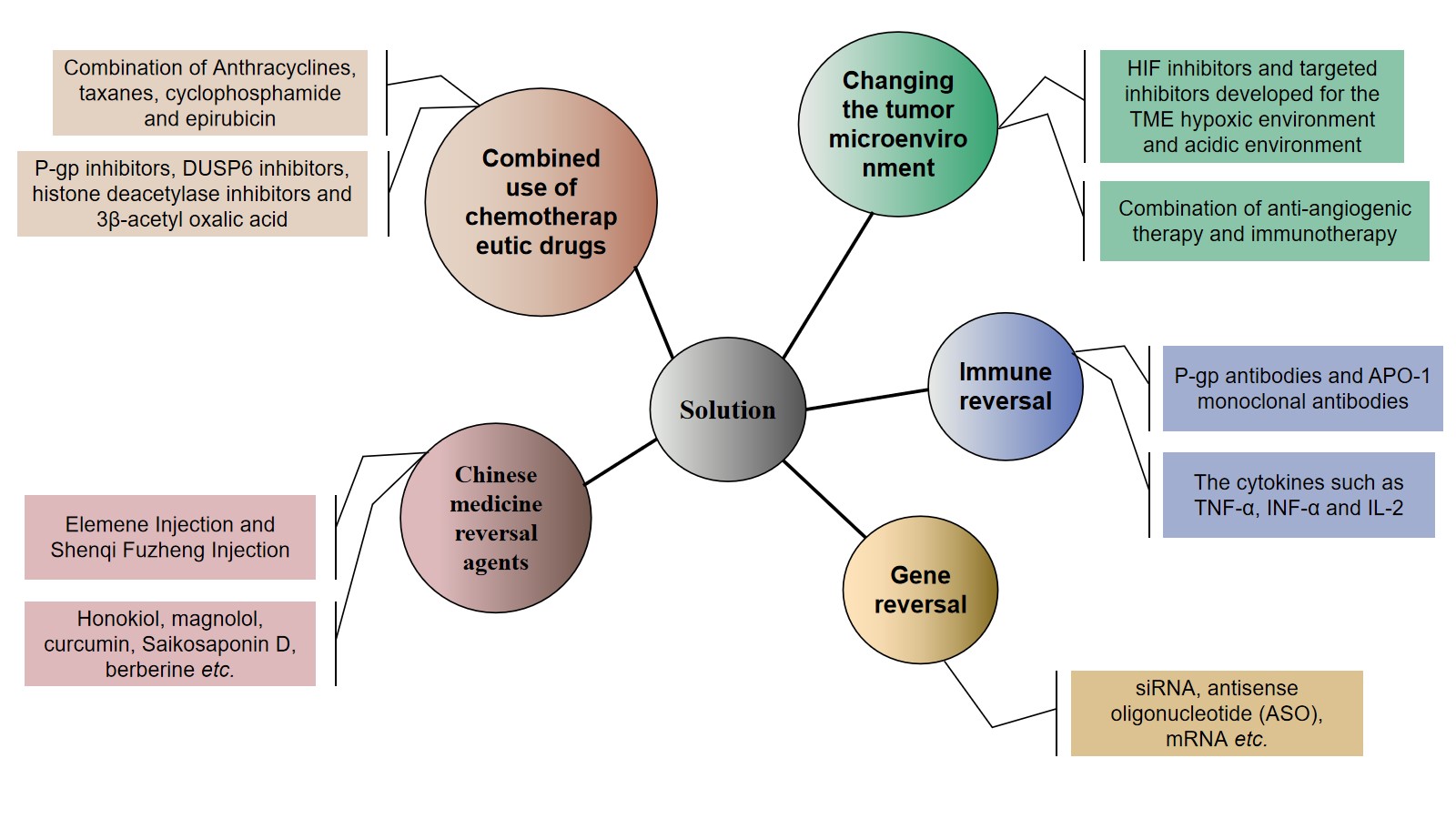
Figure 2. Strategies to combat resistance to neoadjuvant chemotherapy drugs in breast cancer.
References
- Dejana Braithwaite; Joshua Demb; Louise Henderson; Optimal breast cancer screening strategies for older women: current perspectives. Clinical Interventions in Aging 2016, ume 11, 111-125, 10.2147/cia.s65304.
- Freddie Bray; Jacques Ferlay Me; Isabelle Soerjomataram; Rebecca L. Siegel; Lindsey A. Torre; Ahmedin Jemal; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018, 68, 394-424, 10.3322/caac.21492.
- Kara L. Britt; Jack Cuzick; Kelly-Anne Phillips; Key steps for effective breast cancer prevention. Nature Reviews Cancer 2020, 20, 1-20, 10.1038/s41568-020-0266-x.
- Huan Wang; Xiaoyun Mao; Evaluation of the Efficacy of Neoadjuvant Chemotherapy for Breast Cancer. Drug Design, Development and Therapy 2020, ume 14, 2423-2433, 10.2147/dddt.s253961.
- Guoshuang Shen; Fuxing Zhao; Xingfa Huo; Dengfeng Ren; Feng Du; Fangchao Zheng; Jiuda Zhao; Meta-Analysis of HER2-Enriched Subtype Predicting the Pathological Complete Response Within HER2-Positive Breast Cancer in Patients Who Received Neoadjuvant Treatment. Frontiers in Oncology 2021, 11, 632357, 10.3389/fonc.2021.632357.
- Yanding Zhao; Evelien Schaafsma; Chao Cheng; Gene signature‐based prediction of triple‐negative breast cancer patient response to Neoadjuvant chemotherapy. Cancer Medicine 2020, 9, 6281-6295, 10.1002/cam4.3284.
- Tao Hu; Zhen Li; Chun-Ying Gao; Chi Hin Cho; Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World Journal of Gastroenterology 2016, 22, 6876-89, 10.3748/wjg.v22.i30.6876.
- Qi-Nian Wu; Yi-Fu Liao; Yun-Xin Lu; Yun Wang; Jia-Huan Lu; Zhao-Lei Zeng; Qi-Tao Huang; Hui Sheng; Jing-Ping Yun; Dan Xie; et al.Huai-Qiang JuRui-Hua Xu Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Letters 2018, 412, 243-255, 10.1016/j.canlet.2017.10.007.
- Fritz Lai; Lei Jin; Stuart Gallagher; Branka Mijatov; Xu Dong Zhang; Peter Hersey; Histone Deacetylases (HDACs) as Mediators of Resistance to Apoptosis in Melanoma and as Targets for Combination Therapy with Selective BRAF Inhibitors. Advances in Pharmacology 2012, 65, 27-43, 10.1016/b978-0-12-397927-8.00002-6.
- Fu Peng; Liang Xiong; Cheng Peng; (-)-Sativan Inhibits Tumor Development and Regulates miR-200c/PD-L1 in Triple Negative Breast Cancer Cells. Frontiers in Pharmacology 2020, 11, 251, 10.3389/fphar.2020.00251.
- Fu Peng; Hailin Tang; Junrong Du; Jianping Chen; Cheng Peng; Isoliquiritigenin Suppresses EMT-Induced Metastasis in Triple-Negative Breast Cancer through miR-200c/C-JUN/β-Catenin. The American Journal of Chinese Medicine 2021, 49, 505-523, 10.1142/s0192415x21500233.
- Fu Peng; Liang Xiong; Xiaofang Xie; Hailin Tang; Ruizhen Huang; Cheng Peng; Isoliquiritigenin Derivative Regulates miR-374a/BAX Axis to Suppress Triple-Negative Breast Cancer Tumorigenesis and Development. Frontiers in Pharmacology 2020, 11, 378, 10.3389/fphar.2020.00378.
- Li Wang; Fu Peng; Cheng Peng; Jun-Rong Du; Gut Microbiota in Tumor Microenvironment: A Critical Regulator in Cancer Initiation and Development as Potential Targets for Chinese Medicine. The American Journal of Chinese Medicine 2021, 49, 609-626, 10.1142/s0192415x21500270.
- Lan Lin; Lianbin Li; Xiangqi Chen; Bangwei Zeng; Tingyan Lin; Preliminary evaluation of the potential role of β‑elemene in reversing erlotinib‑resistant human NSCLC A549/ER cells. Oncology Letters 2018, 16, 3380-3388, 10.3892/ol.2018.8980.
- Chengcai Yao; Jie Jiang; Yuanrong Tu; Shefang Ye; Haoxin Du; Yi Zhang; β-elemene reverses the drug resistance of A549/DDP lung cancer cells by activating intracellular redox system, decreasing mitochondrial membrane potential and P-glycoprotein expression, and inducing apoptosis. Thoracic Cancer 2014, 5, 304-312, 10.1111/1759-7714.12093.
- Jun Zhang; He-Da Zhang; Yu-Feng Yao; Shan-Liang Zhong; Jian Hua Zhao; Jin Hai Tang; β-Elemene Reverses Chemoresistance of Breast Cancer Cells by Reducing Resistance Transmission via Exosomes. Cellular Physiology and Biochemistry 2015, 36, 2274-2286, 10.1159/000430191.
- Peng Chen; Xuejie Li; Ruonan Zhang; Shuiping Liu; Yu Xiang; Mingming Zhang; Xiaying Chen; Ting Pan; Lili Yan; Jiao Feng; et al.Ting DuanDa WangBi ChenTing JinWengang WangLiuxi ChenXingxing HuangWenzheng ZhangYitian SunGuohua LiLingpan KongXiaohui ChenYongqiang LiZuyi YangQin ZhangLvjia ZhuoXinbing SuiTian Xie Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation.. Theranostics 2020, 10, 5107-5119, 10.7150/thno.44705.
- Jinxu Wang; Xin Tong; Peibo Li; Hui Cao; Weiwei Su; Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. Journal of Ethnopharmacology 2012, 139, 788-795, 10.1016/j.jep.2011.12.019.
- Ying Xiong; Qiuyu Zhao; Liyan Gu; Chunying Liu; Chun Wang; Shenqi Fuzheng Injection Reverses Cisplatin Resistance through Mitofusin-2-Mediated Cell Cycle Arrest and Apoptosis in A549/DDP Cells. Evidence-Based Complementary and Alternative Medicine 2018, 2018, 1-12, 10.1155/2018/8258246.
- Hyo-Kyung Han; Luu Thi Van Anh; Modulation of P-glycoprotein expression by honokiol, magnolol and 4-O-methylhonokiol, the bioactive components of Magnolia officinalis.. Anticancer Research 2012, 32, 4445-4452.
- Chunjie Wen; Lijuan Fu; Jiafeng Huang; Yi Dai; Bin Wang; Ge Xu; Lanxiang Wu; Honghao Zhou; Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin‑resistant breast cancer cells. Molecular Medicine Reports 2019, 19, 5162-5168, 10.3892/mmr.2019.10180.
- Chun Li; Xingang Guan; Haogang Xue; Peng Wang; Manli Wang; Xiaodong Gai; Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells. Pathology - Research and Practice 2017, 213, 848-853, 10.1016/j.prp.2017.01.022.
- Xi Yang; Baixia Yang; Jing Cai; Chi Zhang; Qu Zhang; Liping Xu; Qin Qin; Hongcheng Zhu; Jianxin Ma; Guangzhou Tao; et al.Hongyan ChengXinchen Sun Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1α in vitro and in vivo. Cancer Biology & Therapy 2013, 14, 1068-1073, 10.4161/cbt.26426.
- Yue Pan; Dan Shao; Yawei Zhao; Fan Zhang; Xiao Zheng; Yongfei Tan; Kan He; Jing Li; Li Chen; Berberine Reverses Hypoxia-induced Chemoresistance in Breast Cancer through the Inhibition of AMPK- HIF-1α. International Journal of Biological Sciences 2017, 13, 794-803, 10.7150/ijbs.18969.
- Yonghui Wang; Yana Sui; Yinggang Tao; Gambogic acid increases the sensitivity to paclitaxel in drug‑resistant triple‑negative breast cancer via the SHH signaling pathway. Molecular Medicine Reports 2019, 20, 4515-4522, 10.3892/mmr.2019.10697.
- Dong Li; Xiao-Yi Song; Qing-Xi Yue; Ya-Jun Cui; Miao Liu; Li-Xing Feng; Wan-Ying Wu; Bao-Hong Jiang; Min Yang; Xiao-Bo Qu; et al.Xuan LiuDe-An Guo Proteomic and bioinformatic analyses of possible target-related proteins of gambogic acid in human breast carcinoma MDA-MB-231 cells. Chinese Journal of Natural Medicines 2015, 13, 41-51, 10.1016/s1875-5364(15)60005-x.
- Guo-Qin Jiang; Jun Pan; Jiang-Feng Shang; Zhi-Xue Yang; Ligustrazine induces apoptosis of breast cancer cells in vitro and in vivo. Journal of Cancer Research and Therapeutics 2015, 11, 454-8, 10.4103/0973-1482.147378.
- Jinkuk Kim; Chunguang Hu; Christelle Moufawad El Achkar; Lauren E. Black; Julie Douville; Austin Larson; Mary K. Pendergast; Sara F. Goldkind; Eunjung A. Lee; Ashley Kuniholm; et al.Aubrie SoucyJai VazeNandkishore R. BelurKristina FredriksenIva StojkovskaAlla TsytsykovaMyriam ArmantRenata L. DiDonatoJaejoon ChoiLaura CornelissenLuis M. PereiraErika F. AugustineCasie A. GenettiKira DiesBrenda BartonLucinda WilliamsBenjamin D. GoodlettBobbie L. RileyAmy PasternakEmily R. BerryKelly A. PflockStephen ChuChantal ReedKimberly TyndallPankaj B. AgrawalAlan H. BeggsP. Ellen GrantDavid K. UrionRichard O. SnyderSusan E. WaisbrenAnnapurna PoduriPeter J. ParkAl PattersonAlessandra BiffiJoseph R. MazzulliOlaf BodamerCharles B. BerdeTimothy W. Yu Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. New England Journal of Medicine 2019, 381, 1644-1652, 10.1056/nejmoa1813279.
- Krzysztof Brzezinka; Ekaterina Nevedomskaya; Ralf Lesche; Andrea Haegebarth; Antonius Ter Laak; Amaury E. Fernández-Montalván; Uwe Eberspaecher; Nicolas D. Werbeck; Ursula Moenning; Stephan Siegel; et al.Bernard HaendlerAshley L. EheimCarlo Stresemann Characterization of the Menin-MLL Interaction as Therapeutic Cancer Target. Cancers 2020, 12, 201, 10.3390/cancers12010201.
- Dang Tan Nguyen; Thi Khanh Le; Clément Paris; Chaïma Cherif; Stéphane Audebert; Sandra Oluchi Udu-Ituma; Sébastien Benizri; Philippe Barthélémy; François Bertucci; David Taïeb; et al.Palma Rocchi Antisense Oligonucleotide-Based Therapeutic against Menin for Triple-Negative Breast Cancer Treatment. Biomedicines 2021, 9, 795, 10.3390/biomedicines9070795.
- S. C. Linn; A. H. Honkoop; K. Hoekman; P. Van Der Valk; H. M. Pinedo; Giuseppe Giaccone; p53 and P-glycoprotein are often co-expressed and are associated with poor prognosis in breast cancer. British Journal of Cancer 1996, 74, 63-68, 10.1038/bjc.1996.316.
- K V Chin; Kazumitsu Ueda; I Pastan; M M Gottesman; Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science 1992, 255, 459-462, 10.1126/science.1346476.
- Hui-Yi Loh; Brendan Norman; Kok-Song Lai; Nik Mohd Afizan Nik Abd. Rahman; Noorjahan Banu Mohamed Alitheen; Mohd Azuraidi Osman; The Regulatory Role of MicroRNAs in Breast Cancer. International Journal of Molecular Sciences 2019, 20, 4940, 10.3390/ijms20194940.
- Yuma Yamada; Mai Tabata; Yukari Yasuzaki; Masatoshi Nomura; Atsushi Shibata; Yuta Ibayashi; Yosuke Taniguchi; Shigeki Sasaki; Hideyoshi Harashima; A nanocarrier system for the delivery of nucleic acids targeted to a pancreatic beta cell line. Biomaterials 2014, 35, 6430-6438, 10.1016/j.biomaterials.2014.04.017.
- Sho Okumura; Yu Hirano; Yasuo Komatsu; Stable duplex-linked antisense targeting miR-148a inhibits breast cancer cell proliferation. Scientific Reports 2021, 11, 1-10, 10.1038/s41598-021-90972-3.
- Charles D. Smith; Jack T. Zilfou; XinQun Zhang; Gary R. Hudes; Kenneth D. Tew; Modulation of P-glycoprotein activity by estramustine is limited by binding to plasma proteins. Cancer 1995, 75, 2597-2604, 10.1002/1097-0142(19950515)75:10<2597::aid-cncr2820751030>3.0.co;2-r.
- Takashi Suda; Tomohiro Takahashi; Pierre Golstein; Shigekazu Nagata; Molecular cloning and expression of the fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75, 1169-1178, 10.1016/0092-8674(93)90326-l.
- Ulrike Stein; Wolfgang Walther; Robert H. Shoemaker; Reversal of Multidrug Resistance by Transduction of Cytokine Genes Into Human Colon Carcinoma Cells. JNCI: Journal of the National Cancer Institute 1996, 88, 1383-1392, 10.1093/jnci/88.19.1383.
- Fengkai Li; Shunsuke Kitajima; Susumu Kohno; Akiyo Yoshida; Shoichiro Tange; Soichiro Sasaki; Nobuhiro Okada; Yuuki Nishimoto; Hayato Muranaka; Naoko Nagatani; et al.Misa SuzukiSayuri MasudaTran C. ThaiTakumi NishiuchiTomoaki TanakaDavid A. BarbieNaofumi MukaidaChiaki Takahashi Retinoblastoma Inactivation Induces a Protumoral Microenvironment via Enhanced CCL2 Secretion.. Cancer Research 2019, 79, 3903-3915, 10.1158/0008-5472.CAN-18-3604.
- Marta Truffi; Serena Mazzucchelli; Arianna Bonizzi; Luca Sorrentino; Raffaele Allevi; Renzo Vanna; Carlo Morasso; Fabio Corsi; Nano-Strategies to Target Breast Cancer-Associated Fibroblasts: Rearranging the Tumor Microenvironment to Achieve Antitumor Efficacy. International Journal of Molecular Sciences 2019, 20, 1263, 10.3390/ijms20061263.
- Wuzhen Chen; Lesang Shen; Jingxin Jiang; Leyi Zhang; Zhigang Zhang; Jun Pan; Chao Ni; Zhigang Chen; Antiangiogenic therapy reverses the immunosuppressive breast cancer microenvironment.. Biomarker Research 2021, 9, 59, 10.1186/s40364-021-00312-w.
- Ricky T. Tong; Yves Boucher; Sergey V. Kozin; Frank Winkler; Daniel J. Hicklin; Rakesh K. Jain; Vascular Normalization by Vascular Endothelial Growth Factor Receptor 2 Blockade Induces a Pressure Gradient Across the Vasculature and Improves Drug Penetration in Tumors. Cancer Research 2004, 64, 3731-3736, 10.1158/0008-5472.can-04-0074.




