Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, C. Phenylpropanoid Biosynthetic Pathways. Encyclopedia. Available online: https://encyclopedia.pub/entry/14290 (accessed on 07 February 2026).
Liu C. Phenylpropanoid Biosynthetic Pathways. Encyclopedia. Available at: https://encyclopedia.pub/entry/14290. Accessed February 07, 2026.
Liu, Chen. "Phenylpropanoid Biosynthetic Pathways" Encyclopedia, https://encyclopedia.pub/entry/14290 (accessed February 07, 2026).
Liu, C. (2021, September 17). Phenylpropanoid Biosynthetic Pathways. In Encyclopedia. https://encyclopedia.pub/entry/14290
Liu, Chen. "Phenylpropanoid Biosynthetic Pathways." Encyclopedia. Web. 17 September, 2021.
Copy Citation
The MYB transcription factors (TFs) are evolving as critical role in the regulation of the phenylpropanoid and tanshinones biosynthetic pathway. MYB TFs relate to a very important gene family, which are involved in the regulation of primary and secondary metabolisms, terpenoids, bioactive compounds, plant defense against various stresses and cell morphology.
tanshinones
phenolic acid
plant defense
pdLNLD/ELxiG/S motif
flavonoids
repressor MYB
bioactive compounds
1. Introduction
The compounds derived from phenylpropanoid denote a different class of secondary metabolites, which start from key enzyme phenylalanine. Phenylpropanoid derived metabolites play an important function in plant resistance mechanisms against biotic and abiotic stress, regulate plant growth and development [1][2] and male fertility [3]. Several of these phenylpropanoid derived compounds are considered to be valuable to human welfare and health. MYB protein associated with a big class of transcription factors, which are responsible for the regulation of the biosynthetic pathway of phenylpropanoid resulting compounds [4]. In plants, phenylpropanoid derived secondary metabolites mainly consist of flavonoid, monolignol, stilbenes, terpenoids and different phenolic acid. Many of these compounds play a key role in identified plants, including as UV light protectants, phytoalexins, carotenoids, strengthen the cell wall and signaling molecules [5].
The pigments that provide the different colors to vegetables, fruits, ornamental foliage, leaves, ornamental flowers and seeds are called flavonoids, which provide health benefits to the human and animals [6]. Flavonoids are the secondary metabolites that are broadly distributed in the plant kingdom, which play key roles in plant defense and development. These secondary metabolites can be divided into different groups based on differences in their structure, such as anthocyanin, proanthocyanin, chalcones, flavones, flavonols, flavandiols, isoflavonoids and phlobaphenes [7]. Flavonoids are the most common occurring pigment in plants. Anthocyanins are commonly known as flavonoid compounds providing blues, pink hues, orange, yellow and red colors to flowers, fruits and vegetables. Anthocyanins play very significant physiological and ecological roles in plants. Anthocyanin is most noticeable in young leaves where they defend developing tissues from light stress. Anthocyanins play a key role in seed dispersal and pollination by attracting the pollinator agent in mature fruits and flowers. Proanthocyanin (also known as tannins) provides as significantly roles, such as strengthening the seed coat and stress tolerance in plants [8]. Furthermore, these compounds are concerned in the regulation signaling, when legume are in nodulation process, transportation of auxin and male fertility determination. Moreover, these compounds are involved in plant defense in opposition to stress (biotic and abiotic). These compounds have very imperative values as nutritional and pharmaceutical compounds [9].
Phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), and p-coumaroyl coenzyme A ligase (4CL) are very important key enzymes, which jointly catalyzed stages (First three stages) involved in biosynthesis of compounds, which are derived from phenylpropanoid, as shown in (Figure 1).
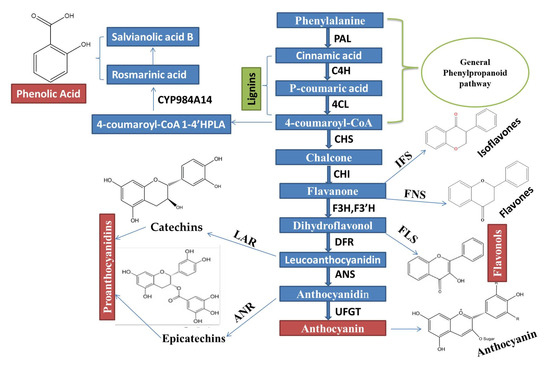
Figure 1. An Integrated regulatory mechanisms network of the Phenylpropanoid biosynthetic pathway, which is controlled through MYB TFs and MBW complex activation, is constructed based on the recent remarkable research advancement.
Various transcription factors (TFs), including R2R3 MYB, WD40 repeat (WDR) proteins and basic helix-loop-helix (bHLH) and control the biosynthesis of flavonoid compounds [10]. A complex of MYB-bHLH-WDR (MBW) shows action, in order to trigger the structural genes responsible for the process of flavonoid biosynthesis. In several plants, including Helianthus annuus L., Arabodopsis thialana, Mimulus guttatus, Camellia sinensis, Narcissus tazetta. L. Narcissus tazetta, Zea mays, Glycine max, Medicago truncatula, Fragaria × ananassa, populous, Petunia x hybrida, Malus domestica, and Vitis vinifera L., these transcription factors have been functionally characterized well [11][12][13][14][15][16][17][18][19].
2. Mechanisms of MYB Gene Family as a Transcription Factor
Cellular processes are regulated by transcription factors (TFs), which can modify complex or intricate traits in plants and could play a prominent part in next-generation biotechnology. There are limitations in genomic diversity in traditional breeding. However, transgenic methodologies surpass genetic obstacles by improving the regulatory pathways of one crop by integrating TFs of other crop or plant species [20]. Genes that encode TFs containing DNA binding motifs, e.g., MYB, ERF/AP2, Zinc fingers and bZIP are signal-induced. These TFs further regulate many functional genes during different conditions of stress or morphogenesis. Therefore, identifying novel TF genes responsible for regulating particular gene expression will improve our understating of signaling pathways related to the development and growth of innovative transgenic crops. MYB is a functionally diverse and large protein family present in all eukaryotic organisms [21]. Many MYB acts as TFs having a different number of MYB domains that are able to bind DNA. They interact with other TFs and are also involved in ABA response, which represents their wide distribution among plants. Detailed functional characterization of these proteins in Arabidopsis thaliana depicts their variety of roles in plant-specific mechanisms. The cell cycle of eukaryotes is controlled by ‘classical’ MYB factors that are linked with c-Myb. First, MYB gene identification was form avian virus myeloblastosis, which was ‘oncogene’ v-MYB [22].
3. Evolution of MYB Transcription Factors
The protein of MYB family contains DNA binding domain. There are two particular conserved regions present in MYB protein, C-terminal of R2R3 MYB protein, which show structural and functional diversity in their amino acid sequence, which are responsible for various regulation activities in plants. While, N-terminal show binding domain of MYB DNA are conserved. Generally, the domain of MYB protein comprises sequences with four imperfect amino acid repeats of approximately 52 amino acids, each establishing three α–helices. Ogata, et al. [23] described that Helix-turn-helix structure, which are built through each repeat of second and third helices with regularly spaced three tryptophan residues, resulting in hydrophobic central in HTH structure (3D). Interestingly, first tryptophan in R3 domain is replaced with isoleucine or phenylalanine in plants. MYB family could be separated into four group based on MYB domain number [2][24]. In plants (monocots and dicots), plentiful kind of R2R3-MYB TFs are specific [25]. The plant taxon represents the highest diversity, with the presence of all four classes of MYB proteins. The group of 4R-MYB indicates the smallest class and its members have four R1/R2-type repeats. Several plant genomes contain single 4R-MYB encoded protein. However, the second class retains 3R-MYB protein of R1R2R3 type, which is composed of higher plant genomes, is particularly encoded by five genes. R2R3-MYB domain is more conserved as compared to its other region, which shows more divergence. The division of R2R3-MYB proteins into subgroups is based on amino acid motifs, which are present at C terminal [2].
MYB domain sequence-based evolutionary studies from various organisms represent that plant ancestor initially had three repeats and out of which the first repeat was lost during the course of time. Lipsick [21] has described an evolutionary model of MYB proteins. This model reveals that R1R2R3-MYBs resulted due to consecutive intragenic duplications and triplications among the primeval eukaryotes, and they produced two repeat and three repeat (R1R2R3-MYB, R2R3-MYB) proteins in animals and plants, respectively. During plant evolution through selective subgroup expansion and amplification, numerous subgroups genes harboring R2R3MYB proteins were made due to the loss of R1 [26]. The consecutive gain of repeat units generated MYB genes. The detailed study of MYB genes regarding their classification, structure, characteristics, mechanism of combinational control, multi-functionality, functional redundancy and gain model for evolution have been reviewed comprehensively by Du, et al. [27] and Dubos, Stracke, Grotewold, Weisshaar, Martin and Lepiniec [24]. It is very interesting that the heterogeneous class consists of proteins with partial or single MYB repeat, jointly known as “MYB-related”, which is further divided into many subclasses [28]. The loss of sequence regarding R1 repeat and successive extension of gene family resulted in R2R3-MYB class after evolution from R1R2-MYB gene predecessor [28].
Moreover, it has also been proposed that ancient intragenic duplication by gaining the sequence encoding R1 repeat from R2R3-MYB genes resulted in the evolution of 3R-MYB [29]. Arabidopsis AtMYB48 and AtMYB59 and their rice homologs (OsMYBAS2 and OsMYBAS1), the two R2R3-MYB genes experience alternative splicing in the same way and result in three diverse merged transcripts in rice, and four in Arabidopsis. Therefore, a deep-rooted understanding of another splicing of MYB protein will further enlighten us regarding gene evolution in dicots and monocots, as well as development-related regulation by transcription factor genes [29].
4. Recent Transcriptomic and Genome-Wide Analysis and Expression of MYB Transcription Factors
In sugarcane, 202 MYB TFs are explored, some of them are expressed mainly in stem and are actively responded to drought stress resistance and mosaic diseases [30]. In Arabidopsis 198 MYBs have been identified; among them, 126 are encoded for R2R3-MYB proteins [31]. Recently, 223 MYB (112 R2R3-MYB, 2 R1R2R3-MYB and 119 R1-MYB) transcription factors were recognized in the potato genome [32]. Recently, there are 69 GbMYB transcription factors are identified in Ginkgo biloba, out of which 19 R2R3 MYB are responsive to hormonal and abiotic stresses [33]. In maize, a genome-wide survey indicated that they consist of 157 R2R3-MYB proteins [34]. R2R3-MYB (185) transcription factors are reported in the genome of Mangrove, 34 MYB gene are mainly expressed in different tissues (root, leaves), which are related to various stresses (salinity and drought) [35]. Hippophae rhamnoides is the rich source of secondary metabolites, which has economic importance regarding medicinal and nutritional values, 161 R2R3–MYB TFs were obtained through its genome-wide analysis [36]. In a recent study, 111 StR2R3-MYB transcription factors are reported in potato [37]. In the genome of flax, 167 R2R3-MYB, 7 R3-MYB, and 1R4-MYB transcription factors have been identified [38]. However, in soybean, 252 total MYBs were identified and account for about 4% of all transcription factors. They consist of two (4R-MYB) genes, six (3R-MYB) proteins and 244 encodings for R2R3-MYB proteins [39]. Genome-wide analysis of apples revealed that they contained 229 MYB transcription factors. Another recent study has explored 251 and 305 MYB TFs from Musa balbisiana and Musa acuminata, respectively by [40].
5. Biological Functions Regulated through MYB Transcription Factors
MYB TFs control many plant-specific processes. By using molecular and genetic analysis the function of MYB proteins have been greatly described among various plant species, like petunia (Petunia hybrida), apple (Malus domestica), poplar (Populus tremuloides), snapdragon (Antirrhinum majus), grapevine (Vitis vinifera L.), maize (Zea mays) and Arabidopsis thaliana [41]. R2R3-MYB TFs have been widely investigated during last decade and their involvement in several processes have been revealed, such as abiotic and biotic stress [42][43], cold tolerance [41], phenylpropanoid metabolism [44][45], trichomes development [46], flower shape [47], cell shape [48], plant defense mechanisms [49][50][51][52], cell wall development [53] and stomatal closure [54].
References
- Wu, Z.; Li, T.; Liu, X.; Yuan, G.; Hou, H.; Teng, N. A novel R2R3-MYB transcription factor LlMYB305 from Lilium longiflorum plays a positive role in thermotolerance via activating heat-protective genes. Environ. Exp. Bot. 2021, 184, 104399.
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456.
- Xiang, X.-J.; Sun, L.-P.; Yu, P.; Yang, Z.-F.; Zhang, P.-P.; Zhang, Y.-X.; Wu, W.-X.; Chen, D.-B.; Zhan, X.-D.; Khan, R.-M.; et al. The MYB transcription factor Baymax1 plays a critical role in rice male fertility. Theor. Appl. Genet. 2020, 134, 453–471.
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant 2015, 8, 689–708.
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61.
- Paauw, M.; Koes, R.; Quattrocchio, F.M. Alteration of flavonoid pigmentation patterns during domestication of food crops. J. Exp. Bot. 2019, 70, 3719–3735.
- Qi, X.; Fang, H.; Chen, Z.; Liu, Z.; Yu, X.; Liang, C. Ectopic Expression of a R2R3-MYB Transcription Factor Gene LjaMYB12 from Lonicera japonica Increases Flavonoid Accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 4494.
- Allan, A.C. Domestication: Colour and Flavour Joined by a Shared Transcription Factor. Curr. Biol. 2019, 29, R57–R59.
- Lu, N.; Rao, X.; Li, Y.; Jun, J.H.; Dixon, R.A. Dissecting the transcriptional regulation of proanthocyanidin and anthocyanin biosynthesis in soybean (Glycine max). Plant Biotechnol. J. 2021, 19, 1429–1442.
- Koes, R.; Verweij, W.; Quattrocchio, F.M. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242.
- Anwar, M.; Wang, G.; Wu, J.; Waheed, S.; Allan, A.C.; Zeng, L. Ectopic Overexpression of a Novel R2R3-MYB, NtMYB2 from Chinese Narcissus Represses Anthocyanin Biosynthesis in Tobacco. Molecules 2018, 23, 781.
- Anwar, M.; Yu, W.; Yao, H.; Zhou, P.; Allan, A.C.; Zeng, L. NtMYB3, an R2R3-MYB from Narcissus, Regulates Flavonoid Biosynthesis. Int. J. Mol. Sci. 2019, 20, 5456.
- Cho, J.-S.; Jeon, H.-W.; Kim, M.-H.; Vo, T.K.; Kim, J.; Park, E.-J.; Choi, Y.-I.; Lee, H.; Han, K.-H.; Ko, J.-H. Wood forming tissue-specific bicistronic expression of PdGA20ox1 and PtrMYB221 improves both the quality and quantity of woody biomass production in a hybrid poplar. Plant Biotechnol. J. 2018, 17, 1048–1057.
- Bai, J.; Sun, F.; Wang, M.; Su, L.; Li, R.; Caetano-Anollés, G. Correction to: Genome-wide analysis of the MYB-CC gene family of maize. Genetica 2019, 147, 11.
- Zhai, R.; Zhao, Y.; Wu, M.; Yang, J.; Li, X.; Liu, H.; Wu, T.; Liang, F.; Yang, C.; Wang, Z.; et al. The MYB transcription factor PbMYB12b positively regulates flavonol biosynthesis in pear fruit. BMC Plant Biol. 2019, 19, 1–11.
- Muñoz, C.; Fanzone, M.; Lijavetzky, D. Transcriptional regulation of the anthocyanin biosynthesis pathway in developing grapevine berries in cultivar ‘Malbec’ by putative R2R3 MYB negative regulators. Sci. Hortic. 2019, 257, 108663.
- Wang, Y.; Zhang, F.; Cui, W.; Chen, K.; Zhao, R.; Zhang, Z. The FvPHR1 transcription factor control phosphate homeostasis by transcriptionally regulating miR399a in woodland strawberry. Plant Sci. 2018, 280, 258–268.
- Li, J.; Liu, H.; Yang, C.; Wang, J.; Yan, G.; Si, P.; Bai, Q.; Lu, Z.; Zhou, W.; Xu, L. Genome-wide identification of MYB genes and expression analysis under different biotic and abiotic stresses in Helianthus annuus L. Ind. Crop. Prod. 2019, 143, 111924.
- Zhang, S.; Chen, Y.; He, X.; Du, J.; Zhang, R.; Ma, Y.; Hu, X.; Zhang, Z.; Chen, Q.; Wan, X. Identification of MYB Transcription Factors Regulating Theanine Biosynthesis in Tea Plant Using Omics-Based Gene Coexpression Analysis. J. Agric. Food Chem. 2020, 68, 918–926.
- Century, K.S.; Reuber, T.L.; Jakob, K.; Ratcliffe, O.J. Selection of transcription factor variants. US Patents 2008/0301836A1, 4 December 2008.
- Lipsick, J.S. One billion years of Myb. Oncogene 1996, 13, 223–235.
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321.
- Ogata, K.; Kanei-Ishii, C.; Sasaki, M.; Hatanaka, H.; Nagadoi, A.; Enari, M.; Nakamura, H.; Nishimura, Y.; Ishii, S.; Sarai, A. The cavity in the hydrophobic core of Myb DNA-binding domain is reserved for DNA recognition and trans-activation. Nat. Genet. 1996, 3, 178–187.
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581.
- Wilkins, O.; Nahal, H.; Foong, J.; Provart, N.J.; Campbell, M.M. Expansion and Diversification of the Populus R2R3-MYB Family of Transcription Factors. Plant Physiol. 2008, 149, 981–993.
- Jin, H.; Martin, C. Multifunctionality and diversity within the plant MYB-gene family. Plant Mol. Biol. 1999, 41, 577–585.
- Du, H.; Zhang, L.; Liu, L.; Tang, X.-F.; Yang, W.-J.; Wu, Y.-M.; Huang, Y.-B.; Tang, Y.-X. Biochemical and molecular characterization of plant MYB transcription factor family. Biochemistry (Moscow) 2009, 74, 1–11.
- Rosinski, J.A.; Atchley, W.R. Molecular Evolution of the Myb Family of Transcription Factors: Evidence for Polyphyletic Origin. J. Mol. Evol. 1998, 46, 74–83.
- Jiang, C.; Gu, X.; Peterson, T. Identification of conserved gene structures and carboxy-terminal motifs in the Myb gene family of Arabidopsis and Oryza sativa L. ssp. indica. Genome Biol. 2004, 5, R46.
- Yuan, Y.; Yang, X.; Feng, M.; Ding, H.; Khan, M.T.; Zhang, J.; Zhang, M. Genome-wide analysis of R2R3-MYB transcription factors family in the autopolyploid Saccharum spontaneum: An exploration of dominance expression and stress response. BMC Genom. 2021, 22, 622.
- Yanhui, C.; Xiaoyuan, Y.; Kun, H.; Meihua, L.; Jigang, L.; Zhaofeng, G.; Zhiqiang, L.; Yunfei, Z.; Xiaoxiao, W.; Xiaoming, Q.; et al. The MYB Transcription Factor Superfamily of Arabidopsis: Expression Analysis and Phylogenetic Comparison with the Rice MYB Family. Plant Mol. Biol. 2006, 60, 107–124.
- Li, X.; Guo, C.; Ahmad, S.; Wang, Q.; Yu, J.; Liu, C.; Guo, Y. Systematic Analysis of MYB Family Genes in Potato and Their Multiple Roles in Development and Stress Responses. Biomolecules 2019, 9, 317.
- Yang, X.; Zhou, T.; Wang, M.; Li, T.; Wang, G.; Fu, F.-F.; Cao, F. Systematic investigation and expression profiles of the GbR2R3-MYB transcription factor family in ginkgo (Ginkgo biloba L.). Int. J. Biol. Macromol. 2021, 172, 250–262.
- Du, H.; Feng, B.-R.; Yang, S.-S.; Huang, Y.-B.; Tang, Y.-X. The R2R3-MYB Transcription Factor Gene Family in Maize. PLoS ONE 2012, 7, e37463.
- Pradhan, S.; Shyamli, P.S.; Suranjika, S.; Parida, A. Genome Wide Identification and Analysis of the R2R3-MYB Transcription Factor Gene Family in the Mangrove Avicennia marina. Agronomy 2021, 11, 123.
- Liu, H.; Zhang, G.; Lv, Z.; Diao, S.; He, C.; Zhang, J. Genome-wide organization and expression profiling of the R2R3-MYB transcription factor family in sea buckthorn (Hippophae rhamnoides L.). Res. Sq. 2020, 18, 501.
- Li, Y.; Lin-Wang, K.; Liu, Z.; Allan, A.C.; Qin, S.; Zhang, J.; Liu, Y. Genome-wide analysis and expression profiles of the StR2R3-MYB transcription factor superfamily in potato (Solanum tuberosum L.). Int. J. Biol. Macromol. 2020, 148, 817–832.
- Tombuloglu, H. Genome-wide identification and expression analysis of R2R3, 3R-and 4R-MYB transcription factors during lignin biosynthesis in flax (Linum usitatissimum). Genomics 2019, 112, 782–795.
- Du, H.; Yang, S.-S.; Liang, Z.; Feng, B.-R.; Liu, L.; Huang, Y.-B.; Tang, Y.-X. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 2012, 12, 106.
- Tan, L.; Ijaz, U.; Salih, H.; Cheng, Z.; Htet, N.N.W.; Ge, Y.; Azeem, F. Genome-Wide Identification and Comparative Analysis of MYB Transcription Factor Family in Musa acuminata and Musa balbisiana. Plants 2020, 9, 413.
- An, J.P.; Wang, X.F.; Zhang, X.W.; Xu, H.F.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353.
- Yu, M.; Man, Y.; Wang, Y. Light- and Temperature-Induced Expression of an R2R3-MYB Gene Regulates Anthocyanin Biosynthesis in Red-Fleshed Kiwifruit. Int. J. Mol. Sci. 2019, 20, 5228.
- Hao, L.; Shi, S.; Guo, H.; Zhang, J.; Li, P.; Feng, Y. Transcriptome analysis reveals differentially expressed MYB transcription factors associated with silicon response in wheat. Sci. Rep. 2021, 11, 1–9.
- Tak, H.; Negi, S.; Ganapathi, T.R. Overexpression of MusaMYB31, a R2R3 type MYB transcription factor gene indicate its role as a negative regulator of lignin biosynthesis in banana. PLoS ONE 2017, 12, e0172695.
- Ma, D.; Constabel, C.P. MYB Repressors as Regulators of Phenylpropanoid Metabolism in Plants. Trends Plant Sci. 2019, 24, 275–289.
- Davis, G.V.; Glover, B. Characterisation of the R2R3 Myb subgroup 9 family of transcription factors in tomato. bioRxiv 2021.
- Abbas, F.; Ke, Y.; Zhou, Y.; Yu, Y.; Waseem, M.; Ashraf, U.; Wang, C.; Wang, X.; Li, X.; Yue, Y.; et al. Genome-Wide Analysis Reveals the Potential Role of MYB Transcription Factors in Floral Scent Formation in Hedychium coronarium. Front. Plant Sci. 2021, 12.
- Lau, S.-E.; Schwarzacher, T.; Othman, R.Y.; Harikrishna, J.A. dsRNA silencing of an R2R3-MYB transcription factor affects flower cell shape in a Dendrobium hybrid. BMC Plant Biol. 2015, 15, 1–14.
- Koter, M.; Święcicka, M.; Matuszkiewicz, M.; Pacak, A.; Derebecka, N.; Filipecki, M. The miRNAome dynamics during developmental and metabolic reprogramming of tomato root infected with potato cyst nematode. Plant Sci. 2018, 268, 18–29.
- Ballester, A.-R.; Norelli, J.; Burchard, E.; Abdelfattah, A.; Levin, E.; González-Candelas, L.; Droby, S.; Wisniewski, M. Transcriptomic Response of Resistant (PI613981–Malus sieversii) and Susceptible (“Royal Gala”) Genotypes of Apple to Blue Mold (Penicillium expansum) Infection. Front. Plant Sci. 2017, 8, 1981.
- Zhou, H.; Lin-Wang, K.; Wang, F.; Espley, R.V.; Ren, F.; Zhao, J.; Ogutu, C.; He, H.; Jiang, Q.; Allan, A.C.; et al. Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation. New Phytol. 2019, 221, 1919–1934.
- Yu, Y.; Guo, D.-L.; Li, G.; Yang, Y.; Zhang, G.; Li, S.; Liang, Z. The grapevine R2R3-type MYB transcription factor VdMYB1 positively regulates defense responses by activating the stilbene synthase gene 2 (VdSTS2). BMC Plant Biol. 2019, 19, 1–15.
- Zhuang, H.; Chong, S.-L.; Borah, P.; Han, X.; Lin, E.; Tong, Z.; Huang, H. Full-Length Transcriptomic Identification of R2R3-MYB Family Genes Related to Secondary Cell Wall Development in Cunninghamia Lanceolata (Chinese Fir). Res. Sq. 2021.
- Li, J.; Zhao, S.; Yu, X.; Du, W.; Li, H.; Sun, Y.; Sun, H.; Ruan, C. Role of Xanthoceras sorbifolium MYB44 in tolerance to combined drought and heat stress via modulation of stomatal closure and ROS homeostasis. Plant Physiol. Biochem. 2021, 162, 410–420.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
17 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




