
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valeria Gasperi | + 4247 word(s) | 4247 | 2021-09-09 06:12:22 | | | |
| 2 | Peter Tang | Meta information modification | 4247 | 2021-09-17 04:14:21 | | |
Video Upload Options
Breast cancer (BC) is the second most common cancer worldwide and the most commonly occurring malignancy in women. There is growing evidence that lifestyle factors, including diet, body weight and physical activity, may be associated with higher BC risk.
1. Introduction
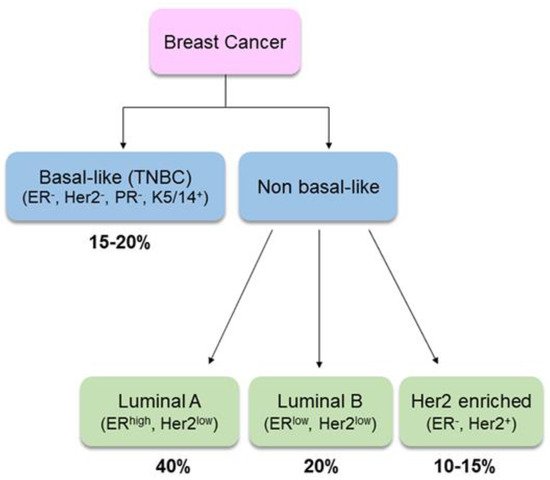
2. Selection of Studies
3. Dietary Factors in Breast Cancer Incidence and Recurrence
|
Study |
Results |
Reference |
|
|---|---|---|---|
|
Fruits, vegetables |
Meta-analysis (15 prospective studies) |
RR = 0.89 (95% CI, 0.80–0.99, p = 0.67) fruits + vegetables; highest vs. lowest intake RR = 0.92 (95% CI, 0.86–0.98, p = 0.36) fruits; highest vs. lowest intake RR = 0.99 (95% CI, 0.92–1.06, p = 0.26) vegetables; highest vs. lowest intake |
[25] |
|
Prospective study (75,929 women, 38–63 years, 24 years follow-up) |
RR = 0.82 (95% CI, 0.71–0.96, p = 0.01), 2 servings/week of total berries RR = 0.69 (95% CI, 0.50–0.95, p = 0.02), 1 serving/week of blueberries RR = 0.59 (95% CI, 0.37–0.93, p = 0.02), 2 servings/week of peaches/nectarines |
[26] |
|
|
Prospective study (31,000 women, 36–64 years, 11.25 years follow-up) |
HR = 0.70 (95% CI, 0.57–0.86, p = 0.0001) leafy vegetables, highest vs. lowest quintile HR = 0.75 (95% CI, 0.60–0.94, p = 0.01) fruiting vegetables, highest vs lowest quintile no association with fruit |
[27] |
|
|
Red meat |
Meta-analysis (13 cohort, 3 case-control, 2 clinical trials) |
RR = 1.06 (95%CI, 0.99–1.14) unprocessed red meat, highest vs. lowest intake RR = 1.09 (95%CI, 1.03–1.16) processed red meat, highest vs. lowest intake |
[28] |
|
Cohort study (262,195 women, 7 years follow-up) Meta-analysis |
HR = 1.21 (95% CI, 1.08–1.35, p = 0.001), >9 g/day processed red meat RR = 1.09 (95% CI 1.03–1.15, p = 0.662), >9 g/day processed red meat in post-menopausal women RR = 0.99 (95% CI 0.88–1.10, p = 0.570), >9 g/day processed red meat in pre-menopausal women |
[29] |
|
|
Dietary Fat |
Randomized controlled trial (48,835 post-menopausal women, 8.1 years follow-up) |
HR = 0.91 (95% CI, 0.83–1.01, NS) intervention group vs. control group |
[30] |
|
Meta-analysis (cohort + case-control studies) |
RR = 1.091 (95% CI, 1.001–1.184) cohort PUFA RR = 1.042 (95%CI, 1.013–1.073) case-control total fat RR = 1.22 (95% CI, 1.08–1.38) case-control PUFA |
[31] |
|
|
Systematic review (18 studies) |
45–78% increased risk of death with increased intake of trans fats |
[32] |
|
|
EPIC study (337,327 women, 11.5 years follow-up) |
HR = 1.20 (95% CI, 1.0–1.45, p = 0.05), highest vs. lowest quintile of total fat intake (ER+PR+ BC) HR = 1.2 (95% CI, 1.09–1.52, p = 0.009), highest vs. lowest quintile of saturated fat intake (ER+PR+ BC) HR = 1.29 (95% CI, 1.01–1.64, p = 0.04), highest vs. lowest quintile of saturated fat intake (HER2− BC) |
[33] |
|
|
Meta-analysis (6 cohort studies + 3 case-control studies) |
RR = 1.29 (95% CI, 1.06–1.56), highest vs. lowest cholesterol intake |
[34] |
|
|
Dairy products |
Pooled analysis (8 prospective cohort studies) (351,041 women, 15 years follow-up) |
NS |
[35] |
|
Meta-analysis (18 prospective cohort studies, n = 1,063,471) |
RR = 0.91 (95% CI, 0.80–1.02, p = 0.003), milk consumption RR = 0.85 (95% CI, 0.76–0.95, p = 0.01), highest vs. lowest total dairy food |
[36] |
|
|
Meta-analysis (22 cohort + 5 case-control studies) |
RR = 0.90 (95% CI, 0.83–0.98, p = 0.111), highest vs. lowest dairy products RR = 0.91 (95% CI, 0.83–0.99, p = 0.991), yogurt consumption RR = 0.85 (95% CI, 0.75–0.96, p = 0.121), low-fat dairy consumption |
[37] |
|
|
Carbohydrate, Glycaemic Index |
Meta-analysis (19 prospective studies) |
RR = 1.04 (95% CI, 1.00–1.07, p = 0.19), 10 units/d for glycemic index RR = 1.01 (95% CI, 0.98–1.04, p = 0.07), 50 units/d for glycemic load RR = 1.00 (95% CI, 0.96–1.05, p = 0.01), 50 g/d for carbohydrate intake |
[38] |
|
Soy products, isoflavones |
Meta-analysis (14 case-control + 7 cohort studies) |
RR = 0.75 (95% CI, 0.59–0.95, p = 0.023), soyfood intake RR = 0.81 (95% CI, 0.67–0.99), isoflavone intake |
[39] |
|
Meta-analysis (1 cohort + 7 case-control studies) |
OR = 0.71 (95% CI, 0.60–0.85, p = 0.023), highest vs. lowest soy intake in Asians OR = 0.88 (95% CI, 0.78–0.98, p = 0.60), moderate vs. lowest soy intake in Asians OR = 1.04 (95% CI, 0.97–1.11, p = 0.42), highest vs. lowest soy isoflavone intake in Western populations |
[40] |
|
|
Meta-analysis (18 prospective studies) |
RR = 0.89 (95% CI, 0.79–0.99, p = 0.001), highest vs. lowest isoflavone intake (RR = 0.76, 95% CI: 0.65–0.86, p = 0.136 in Asian population; RR = 0.97, 95% CI: 0.87–1.06, p = 0.083 in Western population) |
[41] |
4. Impact of Therapy on Nutritional Status of Women with BC
5. Nutritional Interventions during BC Treatment
|
Study |
Intervention |
Results |
Reference |
|
|---|---|---|---|---|
|
ω-3 PUFAs |
Phase II clinical trial (n = 25 breast cancer patients, 31 months follow-up) |
1.8 g DHA/day anthracycline |
Improvement of chemo-therapy outcome: median TTP = 6 months (95% CI, 2.8–8.7 months); median OS = 22 months (95% CI, 17–33 months) No severe adverse side effects (grade 3 or 4 toxicity only for neutropenia and alopecia, 80%) |
[64] |
|
Pilot study (n = 38 postmenopausal breast cancer patients) |
4 g/day EPA + DHA for 3 months AI therapy |
Inhibition of bone resorption in the fish oil responders vs. placebo (p < 0.05) |
[65] |
|
|
Controlled clinical trial (n = 249 postmenopausal breast cancer patients) |
3.3 g/day ω3 PUFA (560 mg EPA + DHA, 40:20 ratio) 24 weeks AI therapy |
Reduction of arthralgia (4.36 vs. 5.70, p = 0.02) obese BC patients vs. placebo |
[66] |
|
|
Controlled clinical trial (n = 20 breast cancer patients) |
EPA (0.19 g/day) + DHA (1.04 g/day) paclitaxel |
Reduction of paclitaxel-induced peripheral neuropathy incidence (OR = 0.3; 95% CI, 0.10–0.88, p = 0.029), but not severity (0.95% CI = (−2.06–0.02), p = 0.054) EPA + DHA vs. placebo |
[67] |
|
|
Green tea |
Prospective cohort study (n = 1160 breast cancer patients, 8 years follow-up) |
Regular consumption of green tea |
Inverse association between regular green tea consumption (≥3 cups/day) and BC recurrence for stage I/II patients (HR = 0.69; 95% CI, 0.47–1.00, p < 0.05) |
[68] |
|
Prospective cohort study (n = 472 breast cancer patients, 7 years follow-up) |
Regular consumption of green tea |
Inverse association between regular green tea consumption (≥5 cups/day) and BC recurrence for stage I/II patients (RR = 0.564; 95% CI, 0.350–0.911, p < 0.05) |
[69] |
|
|
Prospective cohort study (n = 5042, 9.1 years follow-up) |
Regular consumption of green tea |
Reduced risk of total mortality (HR = 0.57; 95% CI: 0.34–0.93) and recurrence (HR = 0.54; 95% CI: 0.31–0.96) for the first 60-month post-diagnosis period |
[70] |
|
|
Vitamin C |
Controlled clinical trial (n = 54 post-menopausal breast cancer patients) |
Vitamin C (500 mg) and E (400 mg) +tamoxifen (10 mg twice a day) for 90 days |
Decrease of total cholesterol, TG, VLDL (p < 0.001) and LDL (p < 0.01) vs. tamoxifen alone Increase of HDL (p < 0.01) vs. tamoxifen alone |
[71] |
|
Controlled clinical trial (n = 40 breast cancer patients) |
Vitamin C (500 mg) and E (400 mg) + 5-fluorouracil (500 mg/m2) + doxorubicin (50 mg/m2) + cyclophosphamide (500 mg/m2) (every 3 weeks for six cycles) |
Increase of SOD, CAT, GST, GPx, GSH (p < 0.01) vs. chemotherapy alone Decrease of MDA, DNA damage (p < 0.01) vs. chemotherapy alone |
[72] |
|
|
Vitamin E |
Prospective cohort study (n = 7 breast cancer patients, 30 days follow-up) |
Vitamin E (400 mg) + tamoxifen (20 mg daily) for 30 days |
Vitamin E supplement interferes with the therapeutic effects of tamoxifen (increase expression of biomarkers of estrogen-stimulation (ER, PR, p-ERK in breast biopsies) |
[73] |
|
Vitamin D |
Prospective cohort study (n = 232 post-menopausal breast cancer patients, 1-year follow-up) |
Calcium (1 g) + vitamin D3 (800 IU/d and additional 16,000 IU, every 2 weeks) + AI therapy for 1 year |
Reduction of AI-associated lumbar spine bone loss: 1.70% (95% CI, 0.4–3.0%; p = 0.005) (women with 25(OH)D serum levels ≥40 ng/ml vs. women with serum levels <30 ng/ml) |
[74] |
|
Prospective cohort study (n = 60 post-menopausal breast cancer patients, 16 weeks follow-up) |
50,000 IU/week + AI therapy for 12 weeks |
Decrease of disability from joint pain (52 vs. 19%; p = 0.026); reduction of fatigue (BFI scores 1.4 vs. 2.9; NS); reduction of menopausal symptoms (MENQOL scores 2.2 vs. 3.2, p = 0.035) (women with 25OHD levels > 66 ng/ml vs. women with levels < 66 ng/ml) |
[75] |
AI: aromatase inhibitor; BC: breast cancer; BFI: big five inventory; CAT: catalase; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; ER: estrogen receptor; GPx: glutathione peroxidase; GSH: reduced glutathione; GST: glutathione transferase; HDL: high density lipoprotein; HR: hazard ratio; LDL: low density lipoprotein; MDA: malondialdehyde; MENQOL: menopause-specific quality of life; NS: not significant; OS: overall survival; p-ERK: phosphorylated extracellular signal–regulated kinase; PR: progesterone receptor; PUFA: poly unsaturated fatty acids; RR: relative risk; SOD: superoxide dismutase; TG: triglycerides; TTP: time to progression; VLDL: very low density lipoprotein; 25OHD: 25-hydroxycholecalciferol.
6. Nutritional Interventions to Reduce BC Recurrence and Mortality
References
- Ferlay, J.; Hery, C.; Autier, P.; Sankaranarayanan, R. Global Burden of Breast Cancer. In Breast Cancer Epidemiology; Springer: New York, NY, USA, 2010; pp. 1–19.
- Seward, B.W.; Wild, C.P. International Agency for Research on Cancer. World Cancer Report 2014; Lyon International Agency for Research on Cancer: Lyon, France, 2014; pp. 16–69.
- Porter, P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N. Engl. J. Med. 2008, 358, 213–216.
- Cancer Statistics Center. Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Breast (accessed on 27 September 2018).
- Global Cancer Observatory. Available online: http://gco.iarc.fr (accessed on 27 September 2018).
- Soerjomataram, I.; Louwman, W.J.; Lemmens, V.E.; de Vries, E.; Klokman, W.J.; Coebergh, J.W. Risks of second primary breast and urogenital cancer following female breast cancer in the south of The Netherlands, 1972–2001. Eur. J. Cancer 2005, 41, 2331–2337.
- Haque, R.; Prout, M.; Geiger, A.M.; Kamineni, A.; Thwin, S.S.; Avila, C.; Silliman, R.A.; Quinn, V.; Yood, M.U. Comorbidities and cardiovascular disease risk in older breast cancer survivors. Am. J. Manag. Care 2014, 20, 86–92.
- Pasanisi, P.; Berrino, F.; De Petris, M.; Venturelli, E.; Mastroianni, A.; Panico, S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int. J. Cancer 2006, 119, 236–238.
- Makari-Judson, G.; Braun, B.; Jerry, D.J.; Mertens, W.C. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J. Clin. Oncol. 2014, 5, 272–282.
- Althuis, M.D.; Fergenbaum, J.H.; Garcia-Closas, M.; Brinton, L.A.; Madigan, M.P.; Sherman, M.E. Etiology of hormone receptor-defined breast cancer: A systematic review of the literature. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1558–1568.
- Anderson, W.F.; Rosenber, P.S.; Prat, A.; Perou, C.M.; Sherman, M.E. How many etiological subtypes of breast cancer: Two, three, four, or more? J. Natl. Cancer Inst. 2014, 106, dju165.
- American Cancer Society. Breast Cancer Facts & Figures 2017–2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf (accessed on 1 June 2019).
- Zare, N.; Haem, E.; Lankarani, K.B.; Heydari, S.T.; Barooti, E. Breast cancer risk factors in a defined population: Weighted logistic regression approach for rare events. J. Breast Cancer 2013, 16, 214–219.
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397.
- Giles, E.D.; Wellberg, E.A.; Astling, D.P.; Anderson, S.M.; Thor, A.D.; Jindal, S.; Tan, A.C.; Schedin, P.S.; Maclean, P.S. Obesity and overfeeding affecting both tumor and systemic metabolism activates the progesterone receptor to contribute to post-menopausal breast cancer. Cancer Res. 2012, 72, 6490–6501.
- Mourouti, N.; Kontogianni, M.D.; Papavagelis, C.; Panagiotakos, D.B. Diet and breast cancer: A systematic review. Int. J. Food Sci. Nutr. 2015, 66, 1–42.
- Protani, M.; Coory, M.; Martin, J.H. Effects of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635.
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2009, 27, 919–926.
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274.
- World Cancer Research Fund, Third Expert Report on “Diet, Nutrition, Physical Activity and Cancer: A Global Perspective”. Available online: https://www.wcrf.org/dietandcancer/breast-cancer (accessed on 27 September 2018).
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48.
- Chan, D.S.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914.
- George, S.M.; Bernstein, L.; Smith, A.W.; Neuhouser, M.L.; Baumgartner, K.B.; Baumgartner, R.N.; Ballard-Barbash, R. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res. Treat. 2014, 146, 647–655.
- Skouroliakou, M.; Grosomanidis, D.; Massara, P.; Kostara, C.; Papandreou, P.; Ntountaniotis, D.; Xepapadakis, G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur. J. Nutr. 2018, 57, 2133–2145.
- Aune, D.; Chan, D.S.; Vieira, A.R.; Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Fruits, vegetables and breast cancer risk: A systematic review and meta-analysis of prospective studies. Breast Cancer Res. Treat. 2012, 134, 479–493.
- Fung, T.T.; Chiuve, S.E.; Willett, W.C.; Hankinson, S.E.; Hu, F.B.; Holmes, M.D. Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among post-menopausal women. Breast Cancer Res. Treat. 2013, 138, 925–930.
- Masala, G.; Assedi, M.; Bendinelli, B.; Ermini, I.; Sieri, S.; Grioni, S.; Sacerdote, C.; Ricceri, F.; Panico, S.; Mattiello, A.; et al. Fruit and vegetables consumption and breast cancer risk: The EPIC Italy study. Breast Cancer Res. Treat. 2012, 132, 1127–1136.
- Farvid, M.S.; Stern, M.C.; Norat, T.; Sasazuki, S.; Vineis, P.; Weijenberg, M.P.; Wolk, A.; Wu, K.; Stewart, B.W.; Cho, E. Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int. J. Cancer 2018, 143, 2787–2799.
- Anderson, J.J.; Darwis, N.D.M.; Mackay, D.F.; Celis-Morales, C.A.; Lyall, D.M.; Sattar, N.; Gill, J.M.R.; Pell, J.P. Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur. J. Cancer 2018, 90, 73–82.
- Prentice, R.L.; Caan, B.; Chlebowski, R.T.; Patterson, R.; Kuller, L.H.; Ockene, J.K.; Margolis, K.L.; Limacher, M.C.; Manson, J.E.; Parker, L.M.; et al. Low fat dietary pattern and risk of invasive breast cancer. The Women’s Health Initiative randomized controlled dietary modification trial. JAMA 2006, 295, 629–642.
- Turner, L.B. A meta-analysis of fat intake, reproduction, and breast cancer risk: An evolutionary perspective. Am. J. Hum. Biol. 2011, 23, 601–608.
- Makarem, N.; Chandran, U.; Bandera, E.V.; Parekh, N. Dietary fat in breast cancer survival. Annu. Rev. Nutr. 2013, 33, 319–348.
- Sieri, S. Dietary fat intake and development of specific breast cancer subtypes. J. Natl. Cancer Inst. 2014, 106, dju068.
- Li, C.; Yang, L.; Zhang, D.; Jiang, W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr. Res. 2016, 36, 627–635.
- Missmer, S.A.; Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; Adami, H.O.; Beeson, W.L.; van den Brandt, P.A.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; et al. Meat and dairy food consumption and breast cancer: A pooled analysis of cohort studies. Int. J. Epidemiol. 2002, 31, 78–85.
- Dong, J.Y.; Zhang, L.; He, K.; Qin, L.Q. Dairy consumption and risk of breast cancer: A meta-analysis of prospective cohort studies. Breast Cancer Res. Treat. 2011, 127, 23–31.
- Zang, J.; Shen, M.; Du, S.; Chen, T.; Zou, S. The association between dairy intake and breast cancer in western and asian populations: A systematic review and meta-analysis. J. Breast Cancer 2015, 18, 313–322.
- Schlesinger, S.; Chan, D.S.M.; Vingeliene, S.; Vieira, A.R.; Abar, L.; Polemiti, E.; Stevens, C.A.T.; Greenwood, D.C.; Aune, D.; Norat, T. Carbohydrates, glycemic index, glycemic load, and breast cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Nutr. Rev. 2017, 75, 420–441.
- Qin, L.Q.; Xu, J.Y.; Wang, P.Y.; Hoshi, K. Soyfood intake in the prevention of breast cancer risk in women: A meta-analysis of observational epidemiological studies. J. Nutr. Sci. Vitam. 2006, 52, 428–436.
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14.
- Dong, J.Y.; Qin, L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323.
- Couto, E.; Sandin, S.; Löf, M.; Ursin, G.; Adami, H.O.; Weiderpass, E. Mediterranean dietary pattern and risk of breast cancer. PLoS ONE 2013, 8, e55374.
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127.
- Castelló, A.; Boldo, E.; Pérez-Gómez, B.; Lope, V.; Altzibar, J.M.; Martín, V.; Castaño-Vinyals, G.; Guevara, M.; Dierssen-Sotos, T.; Tardón, A.; et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 2017, 103, 8–15.
- Toklu, H.; Nogay, N.H. Effects of dietary habits and sedentary lifestyle on breast cancer among women attending the oncology day treatment center at a state university in Turkey. Niger. J. Clin. Pr. 2018, 21, 1576–1584.
- Toledo, E.; Salas-Salvado, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1752–1760.
- Khalis, M.; Chajès, V.; Moskal, A.; Biessy, C.; Huybrechts, I.; Rinaldi, S.; Dossus, L.; Charaka, H.; Mellas, N.; Nejjari, C.; et al. Healthy lifestyle and breast cancer risk: A case-control study in Morocco. Cancer Epidemiol. 2019, 58, 160–166.
- Van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of post-menopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231.
- Fararouei, M.; Iqbal, A.; Rezaian, S.; Gheibi, Z.; Dianatinasab, A.; Shakarami, S.; Dianatinasab, M. Dietary habits and physical activity are associated with the risk of breast cancer among young iranian women: A case-control study on 1010 premenopausal women. Clin. Breast Cancer 2019, 19, 127–134.
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015, 13, 195.
- Kayl, A.E.; Meyers, C.A. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr. Opin. Obs. Gynecol. 2006, 18, 24–28.
- Saquib, N.; Flatt, S.W.; Natarajan, L.; Thomson, C.A.; Bardwell, W.A.; Caan, B.; Rock, C.L.; Pierce, J.P. Weight gain and recovery of pre-cancer weight after breast cancer treatments: Evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res. Treat. 2007, 105, 177–186.
- Buch, K.; Gunmalm, V.; Andersson, M.; Schwarz, P.; Brøns, C. Effect of chemotherapy and aromatase inhibitors in the adjuvant treatment of breast cancer on glucose and insulin metabolism-A systematic review. Cancer Med. 2019, 8, 238–245.
- Caan, B.J.; Kwan, M.L.; Hartzell, G.; Castillo, A.; Slattery, M.L.; Sternfeld, B.; Weltzien, E. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008, 19, 1319–1328.
- Irwin, M.L.; McTiernan, A.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Gilliland, F.D.; Ballard-Barbash, R. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic, and lifestyle factors. J. Clin. Oncol. 2005, 23, 774–782.
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Flatt, S.W.; Lu, W.; Patterson, R.E.; Poole, E.M.; Kwan, M.L.; Chen, Z.; Weltzien, E.; et al. The After Breast Cancer Pooling Project: Rationale, methodology, and breast cancer survivor characteristics. Cancer Causes Control. 2011, 22, 1319–1331.
- Chlebowski, R.T. Nutrition and physical activity influence on breast cancer incidence and outcome. Breast 2013, 22, 30–37.
- Boltong, A.; Aranda, S.; Keast, R.; Wynne, R.; Francis, P.A.; Chirgwin, J.; Gough, K. A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PLoS ONE 2014, 9, e103512.
- De Vries, Y.C.; Boesveldt, S.; Kelfkens, C.S.; Posthuma, E.E.; van den Berg, M.M.G.A.; de Kruif, J.T.C.M.; Haringhuizen, A.; Sommeijer, D.W.; Buist, N.; Grosfeld, S.; et al. Taste and smell perception and quality of life during and after systemic therapy for breast cancer. Breast Cancer Res. Treat. 2018, 170, 27–34.
- De Vries, Y.C.; van den Berg, M.M.G.A.; de Vries, J.H.M.; Boesveldt, S.; de Kruif, J.T.C.M.; Buist, N.; Haringhuizen, A.; Los, M.; Sommeijer, D.W.; Timmer-Bonte, J.H.N.; et al. Differences in dietary intake during chemotherapy in breast cancer patients compared to women without cancer. Support. Care Cancer 2017, 25, 2581–2591.
- Speck, R.M.; DeMichele, A.; Farrar, J.T.; Hennessy, S.; Mao, J.J.; Stineman, M.G.; Barg, F.K. Taste alteration in breast cancer patients treated with taxane chemotherapy: Experience, effect, and coping strategies. Support. Care Cancer 2013, 21, 549–555.
- Murtaza, B.; Hichami, A.; Khan, A.S.; Ghiringhelli, F.; Khan, N.A. Alteration in taste perception in cancer: Causes and strategies of treatment. Front. Physiol. 2017, 8, 134.
- Villarini, A.; Pasanisi, P.; Raimondi, M.; Gargano, G.; Bruno, E.; Morelli, D.; Evangelista, A.; Curtosi, P.; Berrino, F. Preventing weight gain during adjuvant chemotherapy for breast cancer: A dietary intervention study. Breast Cancer Res. Treat. 2012, 135, 581–589.
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1785–1978.
- Hutchins-Wiese, H.L.; Picho, K.; Watkins, B.A.; Li, Y.; Tannenbaum, S.; Claffey, K.; Kenny, A.M. High-dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in post-menopausal breast cancer survivors on aromatase inhibitors: A pilot study. Nutr. Cancer 2014, 66, 68–76.
- Shen, S.; Unger, J.M.; Crew, K.D.; Till, C.; Greenlee, H.; Gralow, J.; Dakhil, S.R.; Minasian, L.M.; Wade, J.L., 3rd; Fisch, M.J.; et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res. Treat. 2018, 172, 603–610.
- Ghoreishi, Z.; Esfahani, A.; Djazayeri, A.; Djalali, M.; Golestan, B.; Ayromlou, H.; Hashemzade, S.; Asghari Jafarabadi, M.; Montazeri, V.; Keshavarz, S.A.; et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: A randomized double-blind placebo controlled trial. Bmc Cancer 2012, 12, 355.
- Inoue, M.; Tajima, K.; Mizutani, M.; Iwata, H.; Iwase, T.; Miura, S.; Hirose, K.; Hamajima, N.; Tominaga, S. Regular consumption of green tea and the risk of breast cancer recurrence: Follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC).; Japan. Cancer Lett. 2001, 167, 175–182.
- Nakachi, K.; Suemasu, K.; Suga, K.; Takeo, T.; Imai, K.; Higashi, Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn. J. Cancer Res. 1998, 89, 254–261.
- Bao, P.P.; Zhao, G.M.; Shu, X.O.; Peng, P.; Cai, H.; Lu, W.; Zheng, Y. Modifiable lifestyle factors and triple-negative breast cancer survival: A population-based prospective study. Epidemiology 2015, 26, 909–916.
- Babu, R.J.; Sundravel, S.; Arumugam, G.; Renuka, R.; Deepa, N.; Sachdanandam, P. Salubrious effect of vitamin C and vitamin E on tamoxifen-treated women in breast cancer with reference to plasma lipid and lipoprotein levels. Cancer Lett. 2000, 151, 1–5.
- Suhail, N.; Bilal, N.; Khan, H.Y.; Hasan, S.; Sharma, S.; Khan, F.; Mansoor, T.; Banu, N. Effect of vitamins C and E on antioxidant status of breast-cancer patients undergoing chemotherapy. J. Clin. Pharm. 2012, 37, 22–26.
- Peralta, E.A.; Brewer, A.T.; Louis, S.; Dunnington, G.L. Vitamin E increases biomarkers of estrogen stimulation when taken with tamoxifen. J. Surg. Res. 2009, 153, 143–147.
- Prieto-Alhambra, D.; Servitja, S.; Javaid, M.K.; Garrigós, L.; Arden, N.K.; Cooper, C.; Albanell, J.; Tusquets, I.; Diez-Perez, A.; Nogues, X. Vitamin D threshold to prevent aromatase inhibitor-related bone loss: The B-ABLE prospective cohort study. Breast Cancer Res. Treat. 2012, 133, 1159–1167.
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2010, 119, 111–118.
- Chlebowski, R.T.; Blackburn, G.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study (WINS). J. Natl. Cancer Inst. 2006, 98, 1767–1776.
- Pierce, J.P.; Natarajan, L.; Caan, B.L.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298.
- McCullough, M.L.; Gapstur, S.M.; Shah, R.; Campbell, P.T.; Wang, Y.; Doyle, C.; Gaudet, M.M. Pre- and postdiagnostic diet in relation to mortality among breast cancer survivors in the CPS-II Nutrition Cohort. Cancer Causes Control. 2016, 27, 1303–1314.
- Kroenke, C.H.; Kwan, M.L.; Sweeney, C.; Castillo, A.; Caan, B.J. High- and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J. Natl Cancer Inst. 2013, 105, 616–623.
- Belle, F.N.; Kampman, E.; McTiernan, A.; Bernstein, L.; Baumgartner, K.; Baumgartner, R.; Ambs, A.; Ballard-Barbash, R.; Neuhouser, M.L. Dietary fiber, carbohydrates, glycemic index, and glycemic load in relation to breast cancer prognosis in the HEAL cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 890–899.
- McEligot, A.J.; Largent, J.; Ziogas, A.; Peel, D.; Anton-Culver, H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in post-menopausal women diagnosed with breast cancer. Nutr. Cancer 2006, 55, 132–140.
- Holmes, M.D.; Chen, W.Y.; Hankinson, S.E.; Willett, W.C. Physical activity’s impact on the association of fat and fiber intake with survival after breast cancer. Am. J. Epidemiol. 2009, 170, 1250–1256.
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443.
- Chi, F.; Wu, R.; Zeng, Y.C.; Xing, R.; Liu, Y.; Xu, Z.G. Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pac. J. Cancer Prev. 2013, 14, 2407–2412.
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Lu, W.; Chen, Z.; Kwan, M.L.; Flatt, S.W.; Zheng, Y.; Zheng, W.; Pierce, J.P.; et al. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012, 96, 123–132.
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079.




