Breast cancer (BC) is the second most common cancer worldwide and the most commonly occurring malignancy in women. There is growing evidence that lifestyle factors, including diet, body weight and physical activity, may be associated with higher BC risk.
1. Introduction
Breast cancer (BC) is the second most common cancer worldwide and the most commonly occurring malignancy in women (22.9% of female cancers), with more than 2 million of new cases diagnosed in 2018 [
1,
2]. Although the incidence is higher in Western Europe and North America, it is rising in developing countries, because of increased life expectancy, urbanization, and the adoption of western lifestyles [
3]. According to the American Cancer Society, the five-year survival rate has improved from 63% in 1960 to 90% at present [
4], thanks to earlier diagnosis with mammogram screening, and improved surgery and adjuvant treatment. Indeed, in 2018, BC death rates have rapidly slowed to 6.6% [
5]. However, survivors are at increased risk of recurrence, even 20 years after the initial diagnosis [
6]; in addition, they show increased risk to gain weight and develop other comorbidities, such as cardiovascular diseases or metabolic disorders [
7,
8,
9].
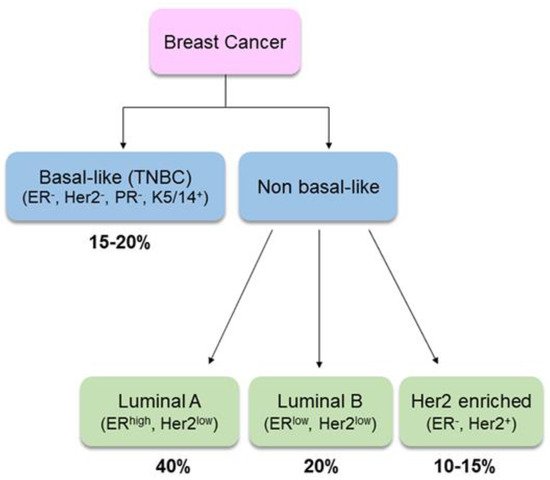
Clinically, BC is a heterogeneous disease. Gene-expression profiling has identified two main groups based on estrogen receptor (ER) expression: ER-expressing (ER
+) breast tumors are more strongly associated with hormone-related factors than tumors that do not express it (ER
−) [
10]. According to cell types of origin (luminal or basal/myoepithelial cell compartment), BC is also classified as basal-like or NON-basal-like. The former, also known as “triple-negative”, accounts for about 10% of all BCs. It is characterized by the absence of all three hormonal receptors, i.e., ER, progesterone receptor (PR) and human growth factor-neu receptor (Her2), while it has high expression of basal cytokeratins. The non-basal like cancer can be further distinguished in luminal A (ER
high/Her2
low), luminal B (ER
low/Her2
low) or Her2-enriched (
Figure 1). Due to the complexity of biology, understanding the etiological heterogeneity of BC subtypes will help in guiding treatment, predicting survival and informing prevention strategies [
11].
Figure 1. Breast cancer sub-types and relative prevalence. TNBC: triple negative breast cancer [
12].
Several risk factors have been identified: non-modifiable factors include older age (>65 versus <65 years), genetic predisposition (including DNA mutations and BC family history), early menarche (<12 years), late menopause (>55 years), age at first pregnancy over 30 years, infertility and not having children, use of contraceptives, hormonal treatment after menopause, and no history of breastfeeding [
13,
14]. Among modifiable lifestyle factors, dietary choices and being overweight or obese are associated with different risks of BC incidence and recurrence [
15,
16]; in particular, obesity is associated with poorer overall survival and increased mortality in post-menopausal BC women [
17].
During recent decades, several studies have evaluated the relationship between specific foods (i.e. alcohol, fruits, vegetables, meat, soy food) and BC development. However, no consistent and statistically strong association has been established, except for alcohol intake [
16]. Nonetheless, it has been proposed that diet may have a significant impact on BC outcomes. Consistent with dietary guidelines directed towards the general population, the adoption of a healthy dietary pattern, based on high consumption of fruits, vegetables, whole grains, poultry and fish, and low consumption of red meat, refined foods, sweets and high-fat dairy products, might improve the overall prognosis and survival of women diagnosed with early-stage BC (stage I, stage II, or stage IIIA) [
18]. Moreover, a growing body of evidence strongly supports that physical activity is also associated with a greater chance of BC surviving [
19].
Based on the most recent evidence, lifestyle recommendations were drawn up by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) [
20]. According to these recommendations, (1) maintaining a healthy body weight, (2) being physically active, (3) following a fiber- and soy-rich diet, and (4) limiting the intake of fats (in particular, saturated fatty acids) may improve overall survival after BC diagnosis [
20]. Much evidence also supports the clinical relevance of nutritional intervention in patients with cancer, aimed at ensuring an adequate intake of energy and nutrients during chemotherapy, which may also result in improved response to and reduced toxicity of pharmacological anti-cancer therapies [
21]. In addition, lifestyle changes including diet and exercise can reduce the long-term side effects of treatment protocols and promote long-term overall health by reducing BC comorbidities (e.g., obesity, hypertension, hyperlipidemia, and diabetes mellitus). Indeed, a potential new role for nutrition as armamentarium of the modern oncologic therapies is emerging.
2. Selection of Studies
A bibliographical search was performed in PubMed using combinations of key words relating to “breast cancer” AND “foodstuffs” (i.e. alcohol, fruits, vegetables, meat, soy food) OR “food nutrients” (i.e. dietary fiber, dietary carbohydrate, glycemic index, dietary fat and fatty acids) OR “fasting” AND “incidence” OR “survival” OR “recurrence” OR “mortality” OR “radiotherapy” OR “chemotherapy” OR “drug side effects”. The eligible criteria include studies in English language published between 2000 and 2019. We included studies referring to either breast cancer incidence, recurrence or survival. In particular, we focused on available meta-analyses and systematic reviews, large epidemiological studies, cohorts and case-control studies and randomized control trials. Information on clinical trials was from URL:
http://clinicaltrials.gov/. Reference lists from selected articles were also manually checked to identify additional relevant reports. Titles and abstract were independently screened by author to determine study eligibility. Hence, we included articles that linked nutritional factors to (a) breast cancer incidence and recurrence; (b) disease-specific mortality or all-cause mortality; (c) breast cancer therapy (d) reduction of drug-related side effects. We considered only prospective cohort studies that had a total sample size of at least 200 subjects (with the only exception for studies relative to vitamin supplementation and drugs side effects). There was no restriction for the menopausal period or the cancer subtype or the type of anti-cancer therapy that patients received.
From the initial search, 361 papers including only humans were returned; among them, 207 articles derived from the combination “breast cancer” AND “foodstuffs” OR “food nutrients” AND “incidence” OR “survival” OR “recurrence” OR “mortality”, while 154 articles derived from the keywords “breast cancer” AND “foodstuffs” OR “food nutrients” AND “radiotherapy” OR “chemotherapy” OR “drug side effects”. After manual screening for duplication and available full-text articles, 249 articles were excluded and a total of 112 pertinent articles were selected for the specific scope of this review.
Additionally, global breast cancer facts and statistics were extracted from the web platforms of leading authorities (i.e. World Cancer Research Fund, International Cancer Societies and World Health Organization).
Finally, some in vitro and in vivo studies were also included to give more insight into the potential mechanism(s) of action underlying the effects observed in humans.
3. Dietary Factors in Breast Cancer Incidence and Recurrence
Adhering to a healthy lifestyle, including weight management and high-quality diet, influences both the risk of developing BC and post-diagnosis outcomes. Mainly, sedentary lifestyle and poor dietary habits, characterized by excessive intake of high-caloric foods (rich in sugar and saturated fats), as well as low intake of healthy foods (containing ω-3 fatty acids, natural antioxidants, fiber), ultimately lead to obesity. Such a condition contributes to increased adipose tissue inflammation, creating a favorable microenvironment for BC development and progression. Indeed, obesity is associated with both increased risk of post-menopausal BC and BC recurrence and mortality. A systematic literature review and meta-analysis of 82 follow-up studies, including 213,075 BC survivors and 41,477 deaths (23,182 deaths attributed to BC), showed a correlation between body mass index (BMI) and BC survival. In particular, an increased risk of 17%, 11% and 8% for overall mortality and 18%, 14% and 29% for BC-specific mortality has been observed for each 5 kg/m
2 BMI increment (i) before BC diagnosis, (ii) less than 12 months after diagnosis and (iii) 12 or more months after diagnosis, respectively [
22]. Besides BMI, some studies also reported a significant positive association between waist-hip ratio and BC mortality, in post-menopausal women [
20,
23].
Based on epidemiological and pre-clinical studies, some foods and nutrients (e.g., carbohydrates, saturated fat, red and processed meats) are considered potential risk factors for BC, as they increase circulating levels of endogenous estrogen, insulin-like growth factor (IGF)-1 and pro-inflammatory cytokines. In contrast, fiber, ω-3 poly unsaturated fatty acids (PUFAs), vitamins C and E, fruits and vegetables may have a protective role by reducing oxidative stress and lowering chronic inflammation (
Table 1) [
24].
Table 1. Possible effects of dietary factors on BC risk.
| |
Study
|
Results
|
Reference
|
|
Fruits, vegetables
|
Meta-analysis (15 prospective studies)
|
RR = 0.89 (95% CI, 0.80–0.99, p = 0.67) fruits + vegetables; highest vs. lowest intake
RR = 0.92 (95% CI, 0.86–0.98, p = 0.36) fruits; highest vs. lowest intake
RR = 0.99 (95% CI, 0.92–1.06, p = 0.26) vegetables; highest vs. lowest intake
|
[25]
|
|
Prospective study (75,929 women, 38–63 years, 24 years follow-up)
|
RR = 0.82 (95% CI, 0.71–0.96, p = 0.01), 2 servings/week of total berries
RR = 0.69 (95% CI, 0.50–0.95, p = 0.02), 1 serving/week of blueberries
RR = 0.59 (95% CI, 0.37–0.93, p = 0.02), 2 servings/week of peaches/nectarines
|
[26]
|
|
Prospective study (31,000 women, 36–64 years, 11.25 years follow-up)
|
HR = 0.70 (95% CI, 0.57–0.86, p = 0.0001) leafy vegetables, highest vs. lowest quintile
HR = 0.75 (95% CI, 0.60–0.94, p = 0.01) fruiting vegetables, highest vs lowest quintile no association with fruit
|
[27]
|
|
Red meat
|
Meta-analysis (13 cohort, 3 case-control, 2 clinical trials)
|
RR = 1.06 (95%CI, 0.99–1.14) unprocessed red meat, highest vs. lowest intake
RR = 1.09 (95%CI, 1.03–1.16) processed red meat, highest vs. lowest intake
|
[28]
|
|
Cohort study (262,195 women, 7 years follow-up) Meta-analysis
|
HR = 1.21 (95% CI, 1.08–1.35, p = 0.001), >9 g/day processed red meat
RR = 1.09 (95% CI 1.03–1.15, p = 0.662), >9 g/day processed red meat in post-menopausal women
RR = 0.99 (95% CI 0.88–1.10, p = 0.570), >9 g/day processed red meat in pre-menopausal women
|
[29]
|
|
Dietary Fat
|
Randomized controlled trial (48,835 post-menopausal women, 8.1 years follow-up)
|
HR = 0.91 (95% CI, 0.83–1.01, NS) intervention group vs. control group
|
[30]
|
|
Meta-analysis (cohort + case-control studies)
|
RR = 1.091 (95% CI, 1.001–1.184) cohort PUFA
RR = 1.042 (95%CI, 1.013–1.073) case-control total fat
RR = 1.22 (95% CI, 1.08–1.38) case-control PUFA
|
[31]
|
|
Systematic review (18 studies)
|
45–78% increased risk of death with increased intake of trans fats
|
[32]
|
|
EPIC study (337,327 women, 11.5 years follow-up)
|
HR = 1.20 (95% CI, 1.0–1.45, p = 0.05), highest vs. lowest quintile of total fat intake (ER+PR+ BC)
HR = 1.2 (95% CI, 1.09–1.52, p = 0.009), highest vs. lowest quintile of saturated fat intake (ER+PR+ BC)
HR = 1.29 (95% CI, 1.01–1.64, p = 0.04), highest vs. lowest quintile of saturated fat intake (HER2− BC)
|
[33]
|
|
Meta-analysis (6 cohort studies + 3 case-control studies)
|
RR = 1.29 (95% CI, 1.06–1.56), highest vs. lowest cholesterol intake
|
[34]
|
|
Dairy products
|
Pooled analysis (8 prospective cohort studies) (351,041 women, 15 years follow-up)
|
NS
|
[35]
|
|
Meta-analysis (18 prospective cohort studies, n = 1,063,471)
|
RR = 0.91 (95% CI, 0.80–1.02, p = 0.003), milk consumption
RR = 0.85 (95% CI, 0.76–0.95, p = 0.01), highest vs. lowest total dairy food
|
[36]
|
|
Meta-analysis (22 cohort + 5 case-control studies)
|
RR = 0.90 (95% CI, 0.83–0.98, p = 0.111), highest vs. lowest dairy products
RR = 0.91 (95% CI, 0.83–0.99, p = 0.991), yogurt consumption
RR = 0.85 (95% CI, 0.75–0.96, p = 0.121), low-fat dairy consumption
|
[37]
|
|
Carbohydrate, Glycaemic Index
|
Meta-analysis (19 prospective studies)
|
RR = 1.04 (95% CI, 1.00–1.07, p = 0.19), 10 units/d for glycemic index
RR = 1.01 (95% CI, 0.98–1.04, p = 0.07), 50 units/d for glycemic load
RR = 1.00 (95% CI, 0.96–1.05, p = 0.01), 50 g/d for carbohydrate intake
|
[38]
|
|
Soy products, isoflavones
|
Meta-analysis (14 case-control + 7 cohort studies)
|
RR = 0.75 (95% CI, 0.59–0.95, p = 0.023), soyfood intake
RR = 0.81 (95% CI, 0.67–0.99), isoflavone intake
|
[39]
|
|
Meta-analysis (1 cohort + 7 case-control studies)
|
OR = 0.71 (95% CI, 0.60–0.85, p = 0.023), highest vs. lowest soy intake in Asians
OR = 0.88 (95% CI, 0.78–0.98, p = 0.60), moderate vs. lowest soy intake in Asians
OR = 1.04 (95% CI, 0.97–1.11, p = 0.42), highest vs. lowest soy isoflavone intake in Western populations
|
[40]
|
|
Meta-analysis (18 prospective studies)
|
RR = 0.89 (95% CI, 0.79–0.99, p = 0.001), highest vs. lowest isoflavone intake (RR = 0.76, 95% CI: 0.65–0.86, p = 0.136 in Asian population; RR = 0.97, 95% CI: 0.87–1.06, p = 0.083 in Western population)
|
[41]
|
RR: multivariable-adjusted relative risk; HR: adjusted hazard ratio; OR: odds ratio; CI: confidence intervals; NS: not significant; PUFA: poly unsaturated fatty acids; ER: estrogen receptor; PR: progesterone receptor; HER2: human growth factor-neu receptor; BC: breast cancer; EPIC: European Prospective Investigation into Cancer and Nutrition.
Adhesion to the Mediterranean diet appears to be inversely linked to BC incidence and mortality, although evidence is still limited [
20,
42,
43,
44]. However, several case-control studies and randomized controlled trials have shown that the higher the adherence levels, the lower the BC incidence levels; indeed, poor diet, together with a sedentary lifestyle (e.g. physical inactivity), can increase the risk of developing BC both in Mediterranean Basin countries [
45,
46,
47] and in countries displaying a tendency for “westernization in diet” [
48,
49].
4. Impact of Therapy on Nutritional Status of Women with BC
Many treatment options employed in BC therapy have been demonstrated to carry long-term toxicities. The therapeutic approaches include different chemotherapeutic agents, alone and/or in combination, as well as radiation, surgery (mastectomy or lumpectomy) or hormonal therapies, depending on the stage. Surgery and radiation therapy, often along with chemo or other drug therapies either before or after surgery, are commonly used to treat BC at stages I to III. Systemic therapy (chemotherapy, hormone therapy and antibody therapy) represents the standard treatment for stage IV BC and for distant recurrence. The most common chemotherapeutic regimes include CMF (cyclophosphamide, methotrexate, 5-flourouracil) or anthracyclines (epirubicin or doxorubicin) that have been demonstrated to reduce mortality by 35% [
85]. Therapy usually runs 3-6 months and is often accompanied by side effects, including nausea, vomiting, loss of appetite, dry mouth and changes in taste or smell perception [
86]. Weight gain is the most common side effect occurring in women receiving chemotherapy, and it is associated with a negative effect on quality of life and survival. As reported in the Women’s Healthy Eating and Living (WHEL), women treated with cytotoxic therapies have 65% increased risk of gaining weight during treatment, compared to women receiving other treatments, such as radiotherapy or hormonal therapy (tamoxifen or aromatase inhibitors) [
87]. Increase in body weight after chemotherapy usually ranges between 1 to 5 kg, and may be associated with changes in body composition with increase in fat mass and loss in muscle mass, also known as sarcopenic obesity. Being overweight or obese during chemotherapy may negatively impact BC prognosis and overall survival, since it can influence other medical conditions, such as diabetes, heart disease, hypertension and hypercholesterolemia [
88,
89]. Weight gain normally occurs when energy intake exceeds energy expenditure. However, in BC patients receiving chemotherapy, caloric intake usually decreases over the first year after diagnosis; therefore, weight gain may not result from overeating, but rather, may be related to lower physical activity and reduced resting metabolic rate. A 50% reduction in activity level can be observed in women subjected to chemotherapy, surgery and radiation, because of the constant fatigue or lack of energy. In addition, chemotherapy often impairs glucose metabolism and induces premature menopause that may influence weight gain and tumor growth pathways in BC patients [
88,
90]. The strongest evidence that weight loss resulting from physical activity is associated with better outcomes for BC patients comes from a big-pooled analysis, the After Breast Cancer Pooling Project (AFCPP), evaluating the post-diagnosis lifestyle factors and outcomes in four prospective cohorts of BC survivors. The study project reported 27% decreased risk of mortality in women who performed at least 10 Metabolic Equivalent per Task (MET)-hours per week, corresponding to 3–5 hours walking/week [
91]. Moreover, cohort analyses and small randomized trials have shown that lifestyle interventions (specific dietary patterns or increased physical activity) significantly reduce secretion of insulin, estrogens, IGF-1 and inflammatory markers [
92]. Thus, maintaining a healthy weight in BC women, by increasing physical activity and decreasing body fat, may be a reasonable intervention to improve prognosis.
Finally, it should be underlined that low BMI (<18.5 kg/m
2) is also associated with poorer prognosis. Indeed, therapy-induced nausea has a substantial impact on eating enjoyment, leading to inadequate energy and essential nutrient intakes, and resulting in malnutrition, reduced compliance with treatment regimens, reduced immunity, emotional distress and negative quality of life [
93,
94,
95]. Although this phenomenon appears to be possibly related to major vulnerability of underweight women to treatment [
89], fortunately, these effects are transient, and recover after the end of chemotherapy.
5. Nutritional Interventions during BC Treatment
Changes in taste during BC treatment are mainly due to damage to taste receptor cells (TRCs) localized on the tongue epithelium and throughout the digestive tract caused by radiation or chemotherapeutic agents. Xerostomia (dry mouth) has also been implicated in taste change, as radiation therapy frequently affects saliva quantity and composition by damaging salivary glands. During chemotherapy, women report altered food preferences for macronutrients, which results in significant lower intake of proteins and fats [
95]. An appropriate nutritional counselling can guide patients to adopt appropriate strategies in order to increase food palatability. For example, adding artificial flavors, eating smaller and more frequent meals, using more condiments, adding something sweet to meats, eating more boiled foods, eating candy before meals, drinking sweetened drinks, using plastic eating utensils, drinking from a straw or cooking in non-metal pots and pans can help to reduce the metallic taste frequently associated with meat. Lemon juice, chewing gum and mint also make meals more pleasant. Moreover, patients should maintain good oral hygiene by brushing their teeth and tongue before meals and using baking soda and salt wash or antibacterial mouthwash, as these may also contribute to changes in taste [
96].
Some chemotherapeutic drugs may cause chelation of zinc and other heavy metals, leading to zinc depletion and contributing to loss of taste. Several clinical trials demonstrated that zinc supplementation might be useful for patients undergoing cancer chemotherapy in improving taste perception. Another valuable aid in reducing taste alteration is represented by amifostine, an organic thiophosphate that antagonizes damage of salivary glands triggered by radiation [
97]. Some foods, including creams prepared with unrefined rice, selected cooked vegetables and vegetable and miso (an essential aminoacid-enriched condiment traditionally added to foods) soups, can prevent gastrointestinal symptoms appearing during chemotherapy [
98]. Cereal creams, for example, avoid the irritating effect on the gut mucosa of a large amount of fibers and, in parallel, provide the nutritional advantage of whole grain cereals, while animal protein intake is usually reduced to prevent acidosis.
Beside limiting drug-induced side effects, some dietary constituents can also enhance therapeutic efficacy, thus improving the quality of life for cancer survivors. In the next paragraphs, we will describe some of the most relevant studies about the effects of specific nutrients on cancer therapy (Table 2).
Table 2. Summary of the evidence on nutritional interventions to enhance BC treatment.
| |
Study
|
Intervention
|
Results
|
Reference
|
|
ω-3 PUFAs
|
Phase II clinical trial (n = 25 breast cancer patients, 31 months follow-up)
|
1.8 g DHA/day anthracycline
|
Improvement of chemo-therapy outcome: median TTP = 6 months (95% CI, 2.8–8.7 months); median OS = 22 months (95% CI, 17–33 months)
No severe adverse side effects (grade 3 or 4 toxicity only for neutropenia and alopecia, 80%)
|
[99]
|
|
Pilot study (n = 38 postmenopausal breast cancer patients)
|
4 g/day EPA + DHA for 3 months AI therapy
|
Inhibition of bone resorption in the fish oil responders vs. placebo (p < 0.05)
|
[100]
|
|
Controlled clinical trial (n = 249 postmenopausal breast cancer patients)
|
3.3 g/day ω3 PUFA (560 mg EPA + DHA, 40:20 ratio) 24 weeks AI therapy
|
Reduction of arthralgia (4.36 vs. 5.70, p = 0.02) obese BC patients vs. placebo
|
[101]
|
|
Controlled clinical trial (n = 20 breast cancer patients)
|
EPA (0.19 g/day) + DHA (1.04 g/day) paclitaxel
|
Reduction of paclitaxel-induced peripheral neuropathy incidence (OR = 0.3; 95% CI, 0.10–0.88, p = 0.029), but not severity (0.95% CI = (−2.06–0.02), p = 0.054) EPA + DHA vs. placebo
|
[102]
|
|
Green tea
|
Prospective cohort study (n = 1160 breast cancer patients, 8 years follow-up)
|
Regular consumption of green tea
|
Inverse association between regular green tea consumption (≥3 cups/day) and BC recurrence for stage I/II patients (HR = 0.69; 95% CI, 0.47–1.00, p < 0.05)
|
[103]
|
|
Prospective cohort study (n = 472 breast cancer patients, 7 years follow-up)
|
Regular consumption of green tea
|
Inverse association between regular green tea consumption (≥5 cups/day) and BC recurrence for stage I/II patients (RR = 0.564; 95% CI, 0.350–0.911, p < 0.05)
|
[104]
|
|
Prospective cohort study (n = 5042, 9.1 years follow-up)
|
Regular consumption of green tea
|
Reduced risk of total mortality (HR = 0.57; 95% CI: 0.34–0.93) and recurrence (HR = 0.54; 95% CI: 0.31–0.96) for the first 60-month post-diagnosis period
|
[105]
|
|
Vitamin C
|
Controlled clinical trial (n = 54 post-menopausal breast cancer patients)
|
Vitamin C (500 mg) and E (400 mg) +tamoxifen (10 mg twice a day) for 90 days
|
Decrease of total cholesterol, TG, VLDL (p < 0.001) and LDL (p < 0.01) vs. tamoxifen alone
Increase of HDL (p < 0.01) vs. tamoxifen alone
|
[106]
|
|
Controlled clinical trial (n = 40 breast cancer patients)
|
Vitamin C (500 mg) and E (400 mg) + 5-fluorouracil (500 mg/m2) + doxorubicin (50 mg/m2) + cyclophosphamide (500 mg/m2) (every 3 weeks for six cycles)
|
Increase of SOD, CAT, GST, GPx, GSH (p < 0.01) vs. chemotherapy alone
Decrease of MDA, DNA damage (p < 0.01) vs. chemotherapy alone
|
[107]
|
|
Vitamin E
|
Prospective cohort study (n = 7 breast cancer patients, 30 days follow-up)
|
Vitamin E (400 mg) + tamoxifen (20 mg daily) for 30 days
|
Vitamin E supplement interferes with the therapeutic effects of tamoxifen (increase expression of biomarkers of estrogen-stimulation (ER, PR, p-ERK in breast biopsies)
|
[108]
|
|
Vitamin D
|
Prospective cohort study (n = 232 post-menopausal breast cancer patients, 1-year follow-up)
|
Calcium (1 g) + vitamin D3 (800 IU/d and additional 16,000 IU, every 2 weeks) + AI therapy for 1 year
|
Reduction of AI-associated lumbar spine bone loss: 1.70% (95% CI, 0.4–3.0%; p = 0.005) (women with 25(OH)D serum levels ≥40 ng/ml vs. women with serum levels <30 ng/ml)
|
[109]
|
|
Prospective cohort study (n = 60 post-menopausal breast cancer patients, 16 weeks follow-up)
|
50,000 IU/week + AI therapy for 12 weeks
|
Decrease of disability from joint pain (52 vs. 19%; p = 0.026); reduction of fatigue (BFI scores 1.4 vs. 2.9; NS); reduction of menopausal symptoms (MENQOL scores 2.2 vs. 3.2, p = 0.035) (women with 25OHD levels > 66 ng/ml vs. women with levels < 66 ng/ml)
|
[110]
|
AI: aromatase inhibitor; BC: breast cancer; BFI: big five inventory; CAT: catalase; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; ER: estrogen receptor; GPx: glutathione peroxidase; GSH: reduced glutathione; GST: glutathione transferase; HDL: high density lipoprotein; HR: hazard ratio; LDL: low density lipoprotein; MDA: malondialdehyde; MENQOL: menopause-specific quality of life; NS: not significant; OS: overall survival; p-ERK: phosphorylated extracellular signal–regulated kinase; PR: progesterone receptor; PUFA: poly unsaturated fatty acids; RR: relative risk; SOD: superoxide dismutase; TG: triglycerides; TTP: time to progression; VLDL: very low density lipoprotein; 25OHD: 25-hydroxycholecalciferol.
6. Nutritional Interventions to Reduce BC Recurrence and Mortality
Several dietary intervention trials have been conducted in BC patients during chemotherapy to improve health outcomes. The two bigger studies were the Women’s Intervention Nutrition Study (WINS) and the WHEL study. The first one was conducted on 2437 post-menopausal women with stage I or II BC receiving standard cancer management. During this trial, the hypothesis that dietary fat reduction improves relapse-free survival rate was tested. In the intervention group, fat intake was reduced from 29.2% to 20.3% of total calories, while maintaining nutritional adequacy. After a median follow-up of 5 years, relapse-free survival was 24% higher in the intervention group than in the normal diet group (30% of total energy from fat). Additionally, the relapse-free survival rate was greater in women with ER
− or/and PR
− disease than in women with receptor-positive disease. Further, significant reduction in body weight of approximately 6 pounds has been observed. However, reducing fat (fats, oils and sweets) necessarily led to make healthier choices; in fact, the percentage of subjects consuming fruit and vegetable increased. Thus, changes in intake of other nutrients besides fats in the intervention group might influenced the risk of BC recurrence [
155]. The second randomized controlled study, the WHEL study, examined a different dietary intervention, in 3080 pre- and post-menopausal patients with early-stage disease. Dietary intervention consisted of increased vegetable servings (five servings/day and 16 oz of vegetable juice), fruit (three servings/day) and fiber (30 g/day) intake and reduced fat intake (15–20% of total calories). BC survivors were counselled with home telephone and cooking classes to support adherence to post-diagnosis diet. In addition, the control group received advice to eat at least five portions of fruit and vegetables each day (five-a-day advice). After 7.3-year follow-up, no evidence that the adoption of a dietary pattern high in vegetables, fruit and fiber and low in fat prevents BC recurrence or death has been observed [
156]. Recently, a prospective study, the Cancer Prevention Study-II Nutrition Cohort (CPS-II Nutrition Cohort), conducted among 4452 BC survivors, evaluated whether pre- or post-diagnostic dietary intake consistent with the American Cancer Society (ACS) recommendations for cancer prevention were associated with BC mortality. While no associations between fruit and vegetable or whole grains intake and BC survival were found, an inverse association was observed with red and processed meat consumption and overall mortality [
157]. In the Life After Cancer Epidemiology (LACE) study, the association among post-diagnosis dairy intake and increased overall mortality among women diagnosed with early-stage invasive BC was evaluated. In first analysis, no statistically significant relation has been found; however, in a second sub-analysis, high-fat dairy intake showed positive correlation with overall mortality and BC-specific mortality. These findings were consistent with the hypothesis that dairy fat intake may increase estrogen levels [
158].
The consumption of dietary fiber in BC survivors and its relationship to prognosis has recently been investigated. In the Health, Eating, Activity, and Lifestyle (HEAL) study (
n = 1183 survivors), fiber intake of >8.8 g/day results inversely associated with BC-specific and overall mortality [
159]. Consistent with these findings, in a separate cohort study (
n = 516 survivors) an inverse association between dietary fiber intake and overall mortality has also been observed [
160]. The Nurses’ Health Study (
n = 3846) reported decreased risk of overall mortality, after initial BC diagnosis, only for cereal fibers [
161], whereas the WHEL trial found no relationship between high fiber intake and BC events or mortality [
156]. Overall, evidence suggests that dietary fiber intake (at least 10 g/day, approximately equivalent to three slices of whole grain bread) significantly decreases risk (about 12%) of all-cause mortality [
18].
Soy food consumption in BC survivors has raised concern about its safety due to the anticancer, but also estrogen-like properties of isoflavones. Epidemiologic data about post-diagnosis soy intake and BC outcomes are insufficient. To date, several studies indicate that soy food intake is inversely associated with mortality and recurrence in Chinese BC women, whereas the evidence is still limited for Western women, for whom soy product consumption is much lower [
41,
162,
163]. A recent pooled analysis on 9514 BC survivors from both US and China showed no significant association between post-diagnosis soy food intake (10 mg isoflavones/day) and reduced risk of all-cause and BC-specific mortality, whereas statistically significant association with reduced recurrence risk has been observed [
164]. Consistent with these findings, a multi-ethnic cohort study of women diagnosed with BC living in North America (17% Hispanics, 12% Blacks, 11% Asian Americans) found a significant trend of lower all-cause mortality associated with higher dietary intake of isoflavones (>10 mg/day). This association has similarly been seen across all racial/ethnic groups, but only in women with negative tumor hormone receptors (ER
−, PR
−) or those not receiving hormonal therapy [
165]. Thus, even if the evidence that post-diagnosis consumption of soy-containing foods reduces the risk of all-cause mortality is limited, it can be considered safe in all women with BC, regardless of hormonal status. In conclusion, a daily reasonable amount of whole soy foods (about 30g, which provides 10-20 mg of soy isoflavones) is potentially beneficial for women with BC, while supplemental soy protein and isoflavone isolates should be avoided.
This entry is adapted from the peer-reviewed paper 10.3390/nu11071514