
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bopaiah Biddanda | + 2341 word(s) | 2341 | 2021-09-02 08:26:54 |
Video Upload Options
Extant microbial mats already present on Earth provide useful working analog models for the exploration of life in extraterrestrial hydrospheres.
1. Background and Context
Earth is a water-dominated planet located in the habitable, or “goldilocks”, zone around the Sun. Indeed, the bulk of Earth’s biosphere is composed of water, wherein the majority of life is microbial—both in terms of numbers and biomass of organisms. Globally distributed microbial mat ecosystems already present underwater on Earth may provide useful models and practical analogs in the search for life in the solar system and beyond where life may exist or have existed as mat worlds.
Evidence of the widespread distribution of microbial mats during the Archean and Proterozoic eons is found today in the geological record of microbialites, such as stromatolites [1]. These modern-day mat ecosystems resemble life during the Precambrian era, when the biosphere was mostly microbial and benthic [2][3][4][5]. Fortunately, even today, microbial mats similar to life in the shallow, anoxic, sometimes euxinic (high hydrogen sulfide, low dissolved oxygen) seas of the early biosphere thrive in several globally distributed refugia under extreme conditions of moisture, salinity, pH, temperature, and oxygen [6][7][8][9][10][11][12][13][14]. Indeed, a great variety of living microbial mats with a diversity of structural and functional characteristics are found in extant extreme ecosystems all over the world ranging from subglacial lakes to deep sea thermal vents [11]. These extremophile microbial mats and their exceptional environments provide readily accessible and working scenarios that could assist us in better envisioning and exploring for life elsewhere in our solar system, such as in Mars, Enceladus and Titan (Saturnian moons), and Ganymede and Europa (Jovian moons) [15].
Herein, we describe life in extant, microbially driven, subsurface mat ecosystems characterized by microbial mats in shallow photic and deep-water aphotic zones and discuss their relevance for exploration of mat worlds that may exist in the solar system and beyond. We do this by first introducing the ecosystems, which are found in the submerged sinkholes of Lake Huron (Michigan, USA): Extensive soft and colorful microbial mats on the sediment surface of submerged sinkholes (~1–200 m) in Lake Huron, a freshwater Laurentian Great Lake (USA).
We follow up with a discussion regarding relevant aspects of how these ecosystems may be useful in studies of potential mat worlds encountered in other extraterrestrial bodies.
2. Microbial Mats in Lake Huron’s Shallow Submerged Sinkholes
Sulfur-rich environments inhabited by cyanobacteria, akin to those that were present long ago in Earth’s history, are found in the coastal waters of NW Lake Huron—a region underlain by karstic limestone wherein groundwater seeps through Paleozoic marine evaporites (Figure 1 and Figure 2) [10]. Here, dissolution of Silurian-Devonian bedrock has produced karstic sinkhole formations on the lake floor through which anoxic, sulfurous groundwater continually vents onto the lake floor, forming a chemocline with the overlying lake water and fueling the growth of microbial mats. The venting groundwater is characterized by nearly constant temperature (9.5 °C), pH (7.3) and oxygen (0–2 mg L −1) and relatively high chloride (25 mg L −1), sulfate (>1000 mg L −1), and conductivity (2300 µS cm −1) compared with overlying Lake Huron water [16]. The denser ground water hugs the bottom of the sinkhole as it flows out, providing a stable hypoxic and sulfur-rich habitat for microbes.
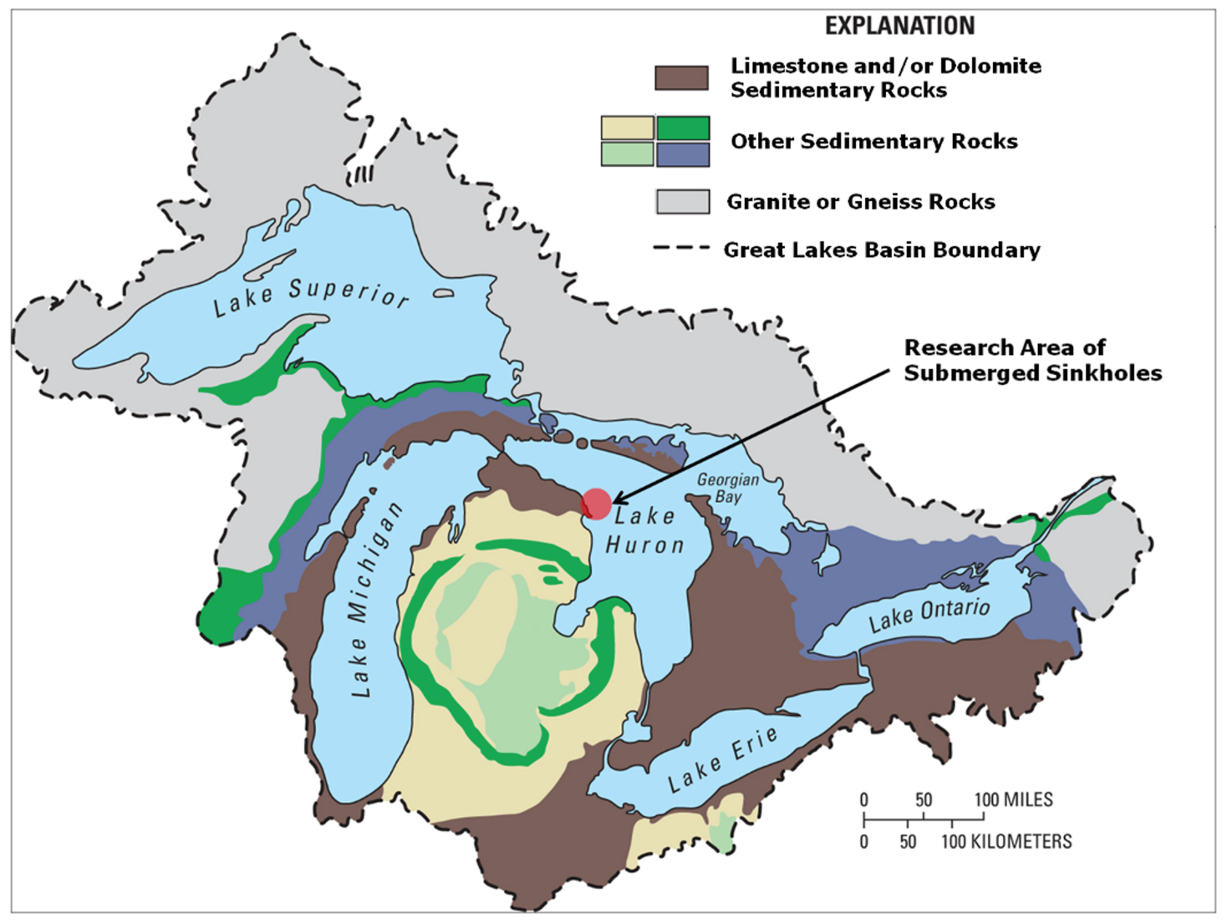
Figure 1. The Great Lakes basin: Surface geology map of the North American Laurentian Great Lakes basin showing abundance of limestone/dolomite bedrock surrounding and underlying the lower Great lakes. Arrow indicates study site in Northwest Lake Huron. Figure from [10], published in Nature Education Knowledge, and originally sourced from Granneman et al. 2000[17] (Cited in the References).
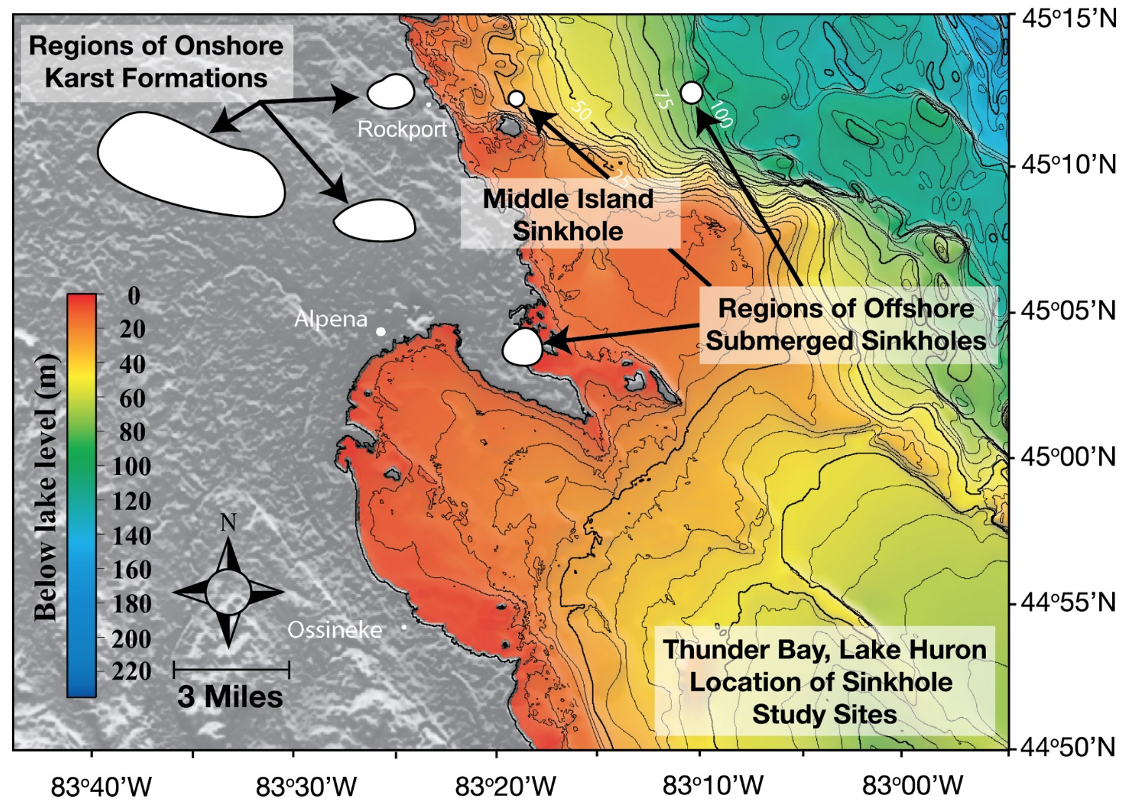
Figure 2. Lake Huron’s submerged sinkholes: Known regions of on-land karst formations in the northeastern lower peninsula of Michigan (USA), and underwater karst sinkholes in the Thunder Bay National Marine Sanctuary, Lake Huron (depth contours in 5m intervals). Site of the present study is the sunlit Middle Island Sinkhole at he 23 M isobath and the aphotic Isolated Sinkhole at the 110m isobath (directly East and offshore of Middle Island Sinkhole). Figure from [10], published in Nature Education Knowledge, created by Cathy Darnell of NOAA-GLERL, and originally sourced from NOAA National Geophysical Data Center 1999 and [18] (Cited in the References).
Microbial mats that cover the floor and walls of these shallow submerged sinkholes are primarily comprised of communities of photosynthetic cyanobacteria and chemosynthetic, sulfur-oxidizing bacteria (SOB; Figure 3 and Figure 4) [10][19]. The cyanobacteria are purple-pigmented and of genera Planktothrix (formerly Oscillatoria ) and Phormidium, which are capable of oxygenic photosynthesis (OP) and anoxygenic photosynthesis (AP), while the SOB are white-appearing and hail from the genus Beggiatoa (Figure 3) [10][19][20]. Successive layers of sulfate-reducing bacteria (SRB) thrive in the organic matter-rich sediments beneath these mats and, together with the producer communities at the top, establish a functioning redox tower [10][21]. These mat ecosystems are closely related and share overall mat morphological similarity to those inhabiting the bottom of terrestrial hot springs distributed worldwide and ice-covered Antarctic lakes [11][22][23], and are analogous to the most ancient forms of life on Earth [24][25]. These features make them model mat systems for exploring the biosphere’s evolution, biodiversity, and physiology under extreme environmental conditions [4][10][26]. It is also likely that microbial mat worlds we may run into in space exploration are analogous to those currently found on Earth. In any case, the diversity of extant mat ecosystems provides the best working models available.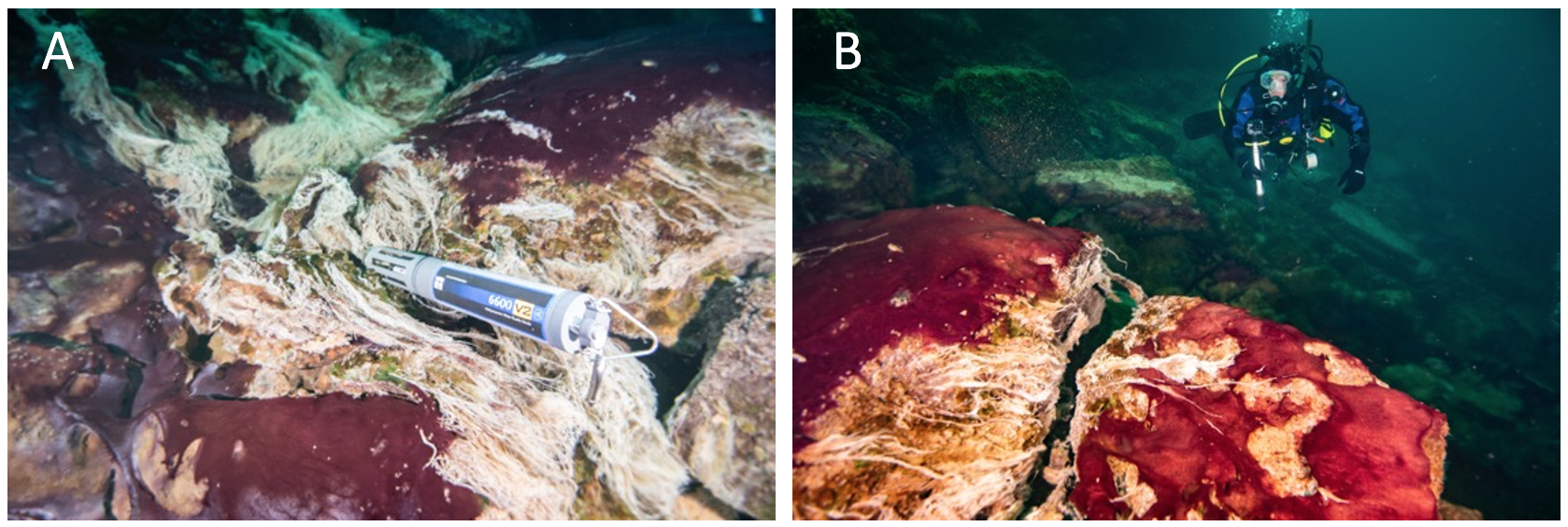
Figure 3. Underwater diver photos showing the predominantly purple-pigmented cyanobacteria along with white patches and strings of chemosynthetic sulfur oxidizing bacteria (note some strings streaming in the low oxygen sulfidic groundwater). A multiparameter water quality sonde (Yellow Springs International) collects time-series environmental data in the groundwater flowing over the mats (A), and a diver uses a GoPro camera to capture images of mats covering the limestone ledge over which groundwater is flowing, fueling extensive microbial growth (B). Scale varies along the photos. For reference, the sonde is ~0.7 m long (A) and the diver is ~0.6 m wide (B).
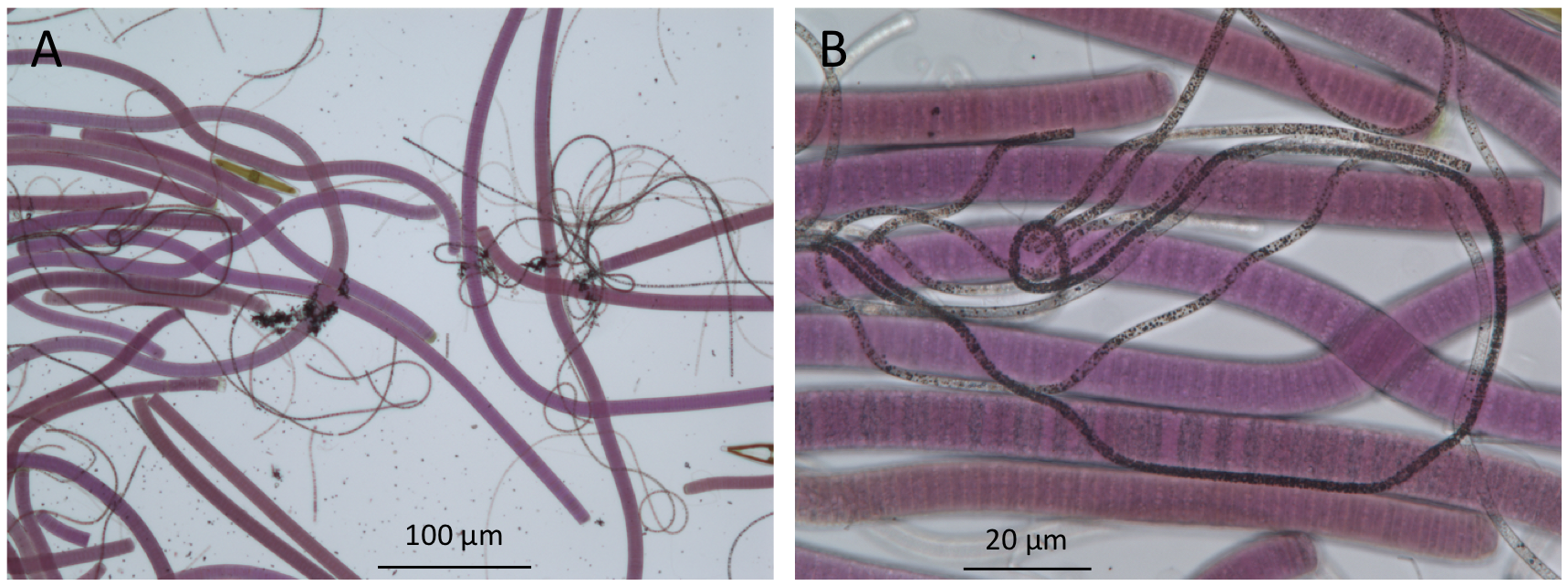
Figure 4. Bright-field microscopy of microbes in the upper millimeters of a mat from Lake Huron’s sinkholes reveal the presence of purple cyanobacteria and filamentous white chemosynthetic bacteria (seen here as dark filaments). (A), low magnification 100× image; (B), high magnification 400× image).
3. Aphotic Mat Worlds—Deep-water Sinkholes of Lake Huron
The study of aphotic ecosystems (those in perpetual darkness - such as deep-sea vents and seeps), presents additional challenges. For example, many varieties of extant microbial mat consortia exist in caves and caverns as well as deep-water ecosystems on Earth where no sunlight is available. At >100 m deep, the Isolated Sinkhole in Lake Huron is one such aphotic ecosystem that is fueled by chemosynthetic microbial mats that thrive on the surface of the sediment, bathed in anoxic and sulfidic ground water (Figure 5) [[27]]. These and other aphotic mat ecosystems (e.g., inside caves, soils, sediments, ice, rocks, etc.) may serve as analogs for mat worlds that are at the far fringes of the solar system and get little sunlight or ones whose sunlight reception is attenuated by the overlying atmosphere/ice/rocks/aqueous media before reaching the live mat zones. However, such aqueous ecosystems should be amenable for robotic exploration when equipped with a light source. Running practice studies with a variety of extant aphotic ecosystems on Earth will be useful in engineering a versatile toolkit capable of effectively exploring similar or different extraterrestrial habitats receiving little to no light from their host sun.
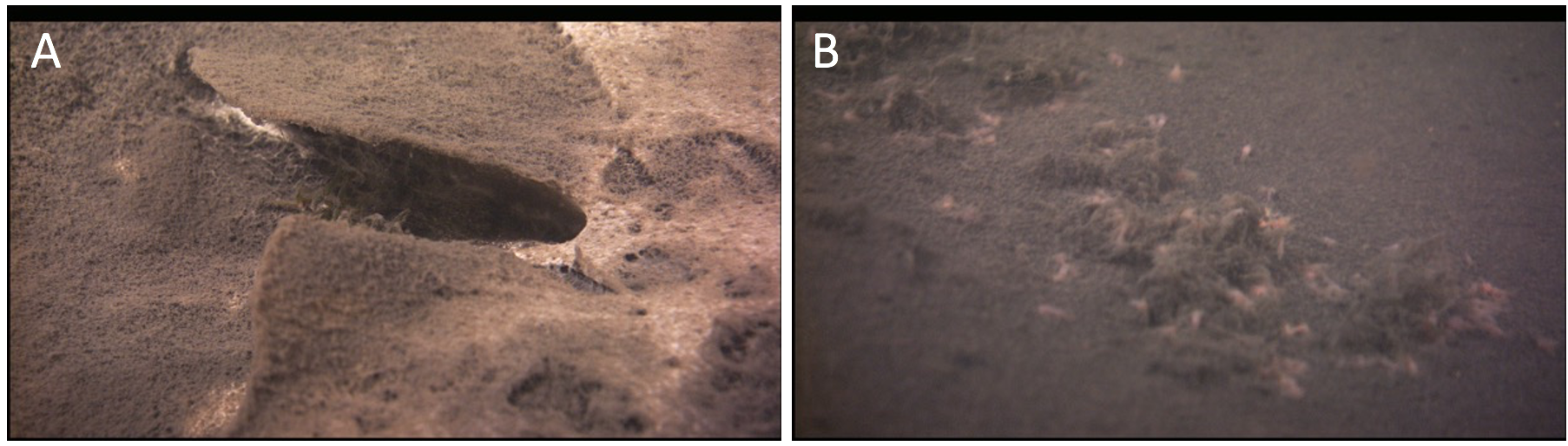
Figure 5. ROV images of the Isolated Sinkhole in Lake Huron, representing a mat world scenario that is under sulfur-rich, anoxic, and aphotic conditions—but one that sustains life in the form of a network of chemosynthetic filaments spread out on the sediment surface in a brown and white layer at a depth of 110 m (A); for scale, the width of the U-shaped depression from which the groundwater is actively venting is ~10 cm; see Biddanda et al. 2006 for more information). Here, in an perpetually dark world, chemosynthetic microbes, such as large sulfur-oxidizing microbes (large pinkish rod-shaped cells in the image), are the primary producers (B); for scale, the large pinkish cells are ~0.5 mm; see [64] for more information).
4. Extant Mat Worlds in Ice and Rock Habitats—From Polar Lakes to Earth’s Deep Biosphere
Antarctica’s permanently frozen subglacial lakes are truly one of Earth’s final frontiers—having been sealed off from the surface for millions of years. Approximately 400 subglacial lakes have been now identified in Antarctica, and sampling of a few lakes suggests that the overlying ice as well as the underlying water are rich in microbes and that the sediment is often overlain by microbial mats [23][28][29][30]. Several studies have measured the metabolism of microbes in these ice-covered lakes and found that a variety of metabolic pathways, ranging from microbial photosynthesis to chemosynthesis, play a key role in the subglacial biogeochemistry of these polar lakes [28][31][32]. Parallels between Antarctica’s subglacial lakes and icecaps of Enceladus and the under-ice ocean of Europa have been noted by others. Microorganisms over-winter in the ice of Antarctic lakes [33], and many have remarked on how the lakes themselves may serve as earthly analog ecosystems in our search for potential life on icy moons, such as Europa, orbiting the outer planets of the solar system [34]. Indeed, several investigators of glacial lakes of Antarctica have suggested that Lake Vostok, with an ice cover of over 3 km and geothermal heating from below, may be a good working analog for the ice-covered oceans of Europa [35][36][37][38].
Earth’s deep biosphere consists of sediments beneath the seafloor and crustal rocks everywhere. Several studies have revealed that the Earth’s living biospshere extends hundreds of meters if not tens of kilometers beneath the continents and the seafloor [39][40]. In these extreme environments, microbial life goes on slowly but surely, using metabolic processes such as carbon oxidation and methanogenesis [41][42]. That microbial cells thrive in sediments hundreds of meters below the seafloor and in Earth’s crustal rocks for more than a kilometer deep suggests various conceivable scenarios in which life could take a foothold and thrive in similarly harsh or harsher extraterrestrial habitats. Indeed, the tolerance of extremophiles, such as methanogens and halophiles, from terrestrial habitats to low temperatures, desiccation, and salinity fluctuations are often considered reasons why extraterrestrial life that we may find on Mars and Europa could resemble those on Earth [43].
Compared to the logistical difficulties of working with microbial mats in the subglacial lakes of the Antarctic or the deep-sea sub-surface biosphere, Lake Huron's submerged sinkholes present amazing working opportunities: Lake Huron's sinkhole ecosystems range from shallow nearshore sunlit ones to deeper light-less ones beyond the 100 m isobath, and have easy access through vessels and infrastructure from an on-site National Marine Sanctuary. Moreover, the nearshore sinkholes become ice-covered during the winter months - presenting a working analog for under-ice habitat exploration - similar to scenarios we may encounter in ice-covered planets. Lastly, most of the known sinkholes being within the boundaries of the National Marine Sanctuary confers them a certain degree of protection from exploitation and destruction - and can thus serve as long-term sites for exploratory science and ecosystem conservation.
5. Extant Earthly Mats as Testbeds for Study of Extraterrestrial Life
Analogs of the simple benthic microbial mats that prevailed in the early biosphere and dominated life for the first few billion years are still found in refugia all around the Earth and could potentially be inhabiting extraterrestrial hydrospheres. For example, geothermal light fuels photosynthetic life in the deep sea [44], microbial life thrives beneath the vast Antarctic shelf [45], and life persists in the deep biosphere within sub-seafloor sediments and even the fluids within crustal rocks [41][42].
The fact that abundant and diverse life occurs hidden in subsurface habitats worldwide makes the case for why we should adapt lessons learned here on Earth in our pursuit for signs and signatures of life in extraterrestrial habitats. In this pursuit, extant Earthly microbial mat communities should serve as useful theoretical and practical models in our search for extraterrestrial life in waters such as those of the subglacial oceans of Europa. This is not to suggest, however, that we have mastered the study of extant extreme environments on this planet and that the field and laboratory methods that work here can be adapted seamlessly for exploring even similar-looking extraterrestrial ecosystems. Surprises are sure to crop up in space, and we will have to adapt by going prepared for it with a versatile tool kit of working methodologies. It is not inconceivable that we will encounter familiar or bizarre mat worlds in our ongoing search for life on habitable moons, planets, and exoplanets—perhaps even witnessing, therein, life’s daily movements synchronized to the tempo of their own planetary spins around their host sun, just as it is here on Earth [46][47]. Our adventures working with earthly extreme ecosystems will have better prepared us to design flexible investigative tools and methodologies to better chronicle such life and their activities if and when we encounter them in space.
Towards the very end of her insightful and witty science book, “The End of Everything: Astrophysically Speaking”, cosmologist Katie Mack writes that, “Like any explorer reaching the edges of the map, we reach out not knowing what we might find” [48]. As Steven Jay Gould observed, “The simplest life may pervade the cosmos” [49], and life’s edge may extend well past our earthly boundaries. Even though we only have evidence of life on Earth so far, “the universe abounds with the chemistry of life” [50]—suggesting that the obvious place to search for extraterrestrial life first is here in our own solar system, where life once formed and may have seeded and reseeded multiple habitable spaces over time [51]. Ongoing and future missions to Mars and the intriguing moons of the outer planets in the solar system offer exciting opportunities for making new discoveries—perhaps finding evidence of past life that is now extinct or even thriving extant extraterrestrial life. Like explorers who have come to the edge of their map, we probe ahead, not knowing what we might find. In the long run, probing for microbial mat worlds that may be out there may be more rewarding than we can imagine.
References
- Stal, L.J. Physiological ecology of cyanobacteria in microbial mats and others communities. New Phytol. 1995, 131, 1–32.
- Schopf, J.W. Precambrian micro-organisms and evolutionary events prior to the origin of vascular plants. Biol. Rev. 1970, 45, 319–352.
- Sanchez-Baracaldo, P. Origin of marine planktonic cyanobacteria. Sci. Rep. 2015, 5, 17418.
- Dick, G.J.; Grim, S.L.; Klatt, J.M. Controls on O2 production in cyanobacterial mats and implications for Earth’s oxygenation. Annu. Rev. Earth Planet. Sci. 2018, 46, 123–147.
- Perez-Ortega, R. Improbable oasis. Science 2020, 369, 20–25.
- Cohen, Y.; Padan, E.; Shilo, M. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limneticai. J. Bacteriol. 1975, 123, 855–861.
- Lugomela, C.; Sderback, E.; Bjork, M. Photosynthesis rates in cyanobacteria-dominated sub-tidal biofilms near Zanzibar, Tanzania. Estuar. Coast. Shelf Sci. 2005, 63, 439–446.
- Jungblut, A.D.; Lovejoy, C.; Vincent, W.F. Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J. 2010, 4, 191–202.
- Yang, T.; Lyons, S.; Aguilar, C.; Cuhel, R.; Teske, A. Microbial community sand chemosynthesis in Yellowstone Lake sublacustrine hydrothermal vent waters. Front. Microbiol. 2011, 2, 130.
- Biddanda, B.A.; Nold, S.C.; Dick, G.J.; Kendall, S.T.; Vail, J.H.; Ruberg, S.A.; Green, C.M. Rock, Water, Microbes: Underwater Sinkholes in Lake Huron are Habitats for Ancient Microbial Life. Nat. Educ. Knowl. 2012, 3, 13.
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56.
- Rosen, M.R.; Arehart, G.B.; Lico, M.S. Exceptionally fast growth rates of <100-yr-old tufa, Big Soda Lake, Nevada: Implications for using tufa as a paleoclimate proxy. Geology 2004, 32, 409–412.
- de Beer, D.; Weber, M.; Chennu, A.; Hamilton, T.; Lott, C.; Macalady, J.; Klatt, J.M. Oxygenic and anoxygenic photosynthesis in a microbial mat from an anoxic and sulfidic spring. Environ. Microbiol. 2017, 19, 1251–1265.
- Zammuto, V.; Rizzo, M.G.; De Plano, L.M.; Franco, D.; Guglielmino, S.; Caccamo, M.T.; Magzaù, S.; Fujimori, A.; Lo Giudice, A.; Guglielmin, M. Effects of heavy ion particle irradiation on spore germination of Bacillus spp. from extremely hot and cold environments. Life 2020, 10, 264.
- Cavicchioli, R. Extremophiles and the search for extraterrestrial life. Int. J. Astrobiol. 2002, 2, 281–292.
- Ruberg, S.A.; Kendall, S.T.; Biddanda, B.A.; Black, T.; Lusardi, W.; Green, R.; Casserley, T.; Smith, E.; Nold, S.; Sanders, T.G.; et al. Observations of the Middle Island sinkhole in Lake Huron: A unique hydrologic and glacial creation of 400 million years. Mar. Technol. Soc. J. 2008, 42, 12–21.
- Grannemann; The importance of groundwater in the Great Lakes region. USGS Water Resources Investigations Report 00-4008 2000, 00-4008, 1-13.
- Coleman, Dwight; Underwater Archaeology in Thunder Bay National Marine Sanctuary, Lake Huron. . Mar Technol Soc J 2002, 36, 33-44.
- Voorhies, A.A.; Biddanda, B.A.; Nold, S.; Kendall, S.; Kain, S.; Marcus, D.; Sheldon, N.; Dick, G. Cyanobacterial life at low O2: Community genomics and function reveal metabolic versatility & extremely low diversity in a Great Lakes sinkhole. Geobiology 2012, 10, 250–267.
- Kinsman-Costello, L.E.; Sheik, C.S.; Sheldon, N.D.; Burton, G.A.; Costello, D.M.; Marcus, D.; Den Uyl, P.A.; Dick, G.J. Groundwater shapes sediment biogeochemistry and microbial diversity in a submerged Great Lake sinkhole. Geobiology 2017, 15, 225–239.
- Nold, S.C.; Pangborn, J.B.; Zajack, H.A.; Kendall, S.T.; Rediske, R.R.; Biddanda, B.A. Benthic bacterial diversity in submerged sinkhole ecosystems. Appl. Environ. Microbiol. 2009, 76, 347–351.
- Castenholz, R.W.; Jørgensen, B.B.; D’Amelio, E.; Bauld, J. Photosynthetic and behavioral versatility of the cyanobacterium Oscillatoria boryana in a sulfide-rich microbial mat. FEMS Microbiol. Ecol. 1991, 9, 43–57.
- Andersen, D.T.; Sumner, D.Y.; Hawes, I.; Webster-Brown, J.; Mcay, C.P. Discovery of large conical stromatolites in Lake Untersee, Antarctica. Geobiology 2011, 9, 280–293.
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the early Archaean era of Australia. Nature 2006, 441, 714–718.
- Falkowski, P.G.; Fenchel, T.; DeLong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039.
- Voorhies, A.A.; Eisenlord, S.D.; Marcus, D.M.; Duhaime, M.B.; Biddanda, B.A.; Cavalcoli, J.D.; Dick, G.J. Ecological and genetic interactions between cyanobacteria and viruses in a low-O2 mat community inferred through metagenomics and metatranscriptomics. Environ. Microbiol. 2016, 18, 358–371.
- Biddanda, B.A.; Coleman, D.F.; Johengen, T.H.; Ruberg, S.A.; Meadows, G.A.; VanSumeren, H.W.; Rediske, R.R.; Kendall, S.T.; Exploration of a submerged sinkhole ecosystem in Lake Huron. . Ecosystems 2006, 9, 828–842, 10.1007/s10021-005-0057-y..
- Vopel, K.; Hawes, I. Photosynthetic performance of benthic microbial mats in Lake Hoare, Antarctica. Limnol. Oceaongr. 2006, 51, 1801–1812.
- Taton, A.; Grubisic, S.; Brambilla, E.; De Wit, R.; Wilmotte, A. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, antarctica): A morphological and molecular approach. Appl. Environ. Microbiol. 2003, 69, 5157–5169.
- Bowman, J.S.; Vick-Majors, T.J.; Morgan-Kiss, R.; Takkacs-Vesbach, C.; Ducklow, H.W.; Priscu, J.C. Microbial community dynamics in tow polar extremes: The lakes of the McMurdo Dy Valleys and the West Antarctic Peninsula marine ecosystem. BioScience 2016, 66, 829–847.
- Mikucki, M.; Priscu, J.C.; Alekhina, I.A.; Wadham, J.L.; Berry-Lyons, W. Subglacial lake Whilhans microbial biogeochemistry: A synthesis of current knowledge. Philos. Trans. R. Soc. A. 2015, 374, 20140466.
- Achberger, A.M.; Christner, B.C.; Michaud, A.B.; Priscu, J.C.; Skidmore, M.L.; Vick-Majors, T.J.; WISSARD Science Team. Microbial community structure of subglacial Lake Whilhans, West Antarctica. Front. Microbiol. 2016, 7, 1457.
- Foreman, C.M.; Dieser, M.; Greenwood, M.; Cory, R.M.; Laybourn-Parry, J.; Lisle, J.T.; Jaros, C.; Miller, P.L.; Chin, Y.-P.; McKnight, D.M. When habitat freezes solid: Microorganisms over-winter within the ice column of a coastal Antarctic lake. FEMS Microbiol. Ecol. 2011, 76, 401–412.
- Dudeja, S.; Bhattacherjee, A.B.; Chela-Flores, J. Microbial mats in Antarctica as models for the search of life on the Jovian moon Europa. In Microbial Mats; COLE Series; Springer: Dordrecht, The Netherlands, 2010; pp. 543–561.
- Siegert, M.J.; Ellis-Evans, J.C.; Tranter, M.; Mayer, C.; Petit, J.R.; Salamatin, A.; Priscu, J.C. Physical, chemical and biological processes in Lake Vostok and other Antarctic subglacial lakes. Nature 2001, 414, 603–609.
- Priscu, J.C.; Bell, R.E.; Bulat, S.A.; Ellis-Evans, C.J.; Kennicutt, M.C.; Lukin, V.V.; Petit, J.-R.; Powell, R.D.; Siegert, M.J.; Tabacco, I. An international plan for Antarctica subglacial lake exploration. Polar Geogr. 2003, 27, 69–83.
- Christner, B.C.; Roysto-Bishop, G.; Foreman, C.M.; Arnold, B.R.; Tranter, M.; Welh, K.A.; Lyons, W.B.; Tsapin, A.I.; Studinger, M.; Priscu, J.C. Limnological conditions in subglacial Lake Vostok, Antarctica. Limnol. Oceanogr. 2006, 51, 2485–2501.
- Dudeja, S.; Bhattacharya, A.B.; Chela-Flores, J. Antarctica as model for the possible emergence of life on Europa. In Life on Earth and Other Planetary Bodies; Hanslmeier, A., Kempe, S., Seckbach, J., Eds.; Cellular Origin and Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2012; pp. 409–422.
- Kallemeyer, J.; Pockalny, R.; Adhikari, R.R.; Smith, D.C.; D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl. Acad. Sci. USA 2012, 109, 16213–16216.
- Parkes, R.J.; Cragg, B.; Roussel, E.; Webster, G.; Weightman, A.; Sass, H. A review of prokaryotic populations and processes in sub-seafloor sediments, including biosphere: Geosphere interactions. Mar. Geol. 2014, 352, 409–425.
- Heuer, V.B.; Inagaki, F.; Morono, Y.; Kubo, Y.; Spivack, A.J.; Viehweger, B.; Treude, T.; Beulig, F.; Schubotz, F.; Tonai, S.; et al. Temperature limits to deep subseafloor life in the Nankai Trough subduction zone. Science 2020, 370, 1230–1234.
- Trembath-Reichert, E.; Sah Walter, S.R.; Fontanez Otitz, M.A.; Carter, P.D.; Girguls, P.R.; Huber, J.A. Multiple carbon incorporation strategies support microbial survival in cold subseafloor crustal fluids. Sci. Adv. 2021, 7, eabg0153.
- Reid, I.N.; Sparks, W.B.; Lubow, S.; McGrath, M.; Livio, M.; Sowers, K.R.; Shukla, H.D.; MacAuley, S.; Miller, T.; Suvanasuthi, R. Terrestrial models for extraterrestrial life: Methanogens and halophiles at Martian temperatures. Int. J. Astrobiol. 2006, 5, 89–97.
- Beatty, J.T.; Overmann, J.; Lince, M.T.; Manske, A.K.; Lang, A.S.; Blankenship, R.E.; Van Dover, C.L.; Martinson, T.A.; Plumley, F.G. An obligately photosynthetic bacterial anaerobe form a deep-sea hydrothermal vent. Proc. Natl. Acad. Sci. USA 2005, 102, 9306–9310.
- Vick-Majors, T.J.; Michaud, A.B.; Skidmore, M.L.; Turetta, C.; Barbante, C.; Christner, B.C.; Dore, J.E.; Christianson, K.; Mitchell, A.C.; Achberger, A.M.; et al. Biogeochemical connectivity between freshwater ecosystems beneath the West Antarctic Ice Sheet and he sub-ice marine environment. Glob. Biogeochem. Cycles 2020, 34, e2019GB006446.
- Biddanda, B.A.; Stone, I.P.; Weinke, A.D. Extant mat world microbes synchronize vertical migration to a diel tempo. Biogeochemistry 2021. under review.
- Klatt, J.M.; Chennu, A.; Arbic, B.K.; Biddanda, B.A.; Dick, G.J. Possible link between Earth’s rotation rate and oxygenation. Nat. Geosci. 2021, 14, 564–570.
- Mack, K. The End of Everything: Astrophysically Speaking; Scribner: New York, NY, USA, 2020; p. 227.
- Gould, S.J. Leonardo’s Mountain of Clams and the Diet of Worms; Harmony Books: New York, NY, USA, 1998; p. 422.
- Sagan, C. The Search for Extraterrestrial Life; Scientific American, Springer Nature: New York, NY, USA, 1994; Volume 271, pp. 92–99.
- Pace, N.R. The universal nature of biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808.




