Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alberto Zafra-Gómez | + 2849 word(s) | 2849 | 2021-08-25 07:55:45 | | | |
| 2 | Peter Tang | Meta information modification | 2849 | 2021-09-03 05:02:02 | | | | |
| 3 | Peter Tang | Meta information modification | 2849 | 2021-09-06 06:06:05 | | | | |
| 4 | Peter Tang | Meta information modification | 2849 | 2021-09-06 06:54:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zafra-Gómez, A. Bisphenol A Analogues in Food. Encyclopedia. Available online: https://encyclopedia.pub/entry/13864 (accessed on 07 February 2026).
Zafra-Gómez A. Bisphenol A Analogues in Food. Encyclopedia. Available at: https://encyclopedia.pub/entry/13864. Accessed February 07, 2026.
Zafra-Gómez, Alberto. "Bisphenol A Analogues in Food" Encyclopedia, https://encyclopedia.pub/entry/13864 (accessed February 07, 2026).
Zafra-Gómez, A. (2021, September 02). Bisphenol A Analogues in Food. In Encyclopedia. https://encyclopedia.pub/entry/13864
Zafra-Gómez, Alberto. "Bisphenol A Analogues in Food." Encyclopedia. Web. 02 September, 2021.
Copy Citation
Bisphenol A (BPA) is the most well-known compound from the bisphenol family. As BPA has recently come under pressure, it is being replaced by compounds very similar in structure.
bisphenol A analogues
food
obesogenic effect
1. Introduction
Endocrine disruptors are compounds that alter the normal functioning of the endocrine system, and their bioaccumulation in humans may cause adverse health effects [1][2][3]. Bisphenol A (BPA) is a well-known endocrine disruptor, industrially produced, largely used as a component of epoxy resins and polycarbonate plastics [4][5]. BPA-based plastics and resins are used in the manufacturing of food contact material such as packaging, crockery, and thermic paper. Human exposure to BPA occurs mainly through diet (food and food contact materials) [6]. Moreover, BPA has also been found in plastic food containers, epoxy coatings in metal cans, kitchenware toys, medical devices, and dental composites and sealants [4][7][8][9][10].
In humans, BPA has proven to have developmental, reproductive, cardiovascular, immune, and metabolic effects [11]. In 2017, BPA was listed in the substances of very high concern list of the European Chemical Agency (ECHA). In view of the recent regulations that further restrict the use of BPA in food contact materials [12][13][14][15][16], food packaging companies are exploring substitutes to gradually eliminate BPA from their products [10][17][18].
Commercialization of BPA-free labeled products is increasing, while BPA analogues are being increasingly used in the manufacturing of consumer products [10]. BPA analogues share the basic bisphenol structure of two benzene rings separated by a short carbon or other chemical chain [10] (Figure 1). Bisphenol S (BPS), bisphenol F (BPF), bisphenol B (BPB), bisphenol E (BPE), and bisphenol AF (BPAF) are chosen by the industry as a replacement for BPA in the production of polycarbonates and epoxy resins [10][19][20] for the manufacturing of industrial and consumer products [21][22]. There are a limited number of studies on the BPA analogues’ hormonal effects, but most show that they have similar health concerns as BPA [23][24][25][26]. In 2002, we demonstrated the endocrine-disrupting activity of BPA analogues in the expression of estrogen-controlled genes [27]. Furthermore, some of the BPA substitutes seem to have more estrogenic effects than BPA [28].
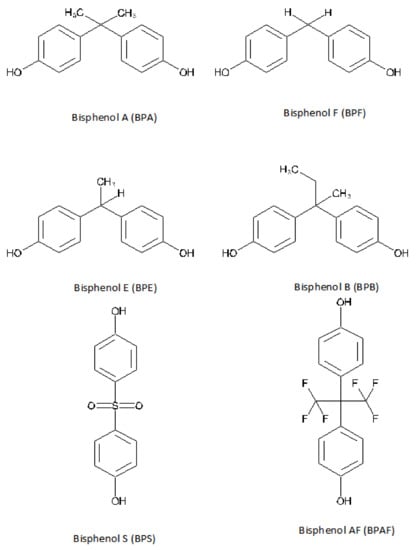
Figure 1. Bisphenols with endocrine-disrupting activity. Prepared by author based on Gallart-Ayala et al., 2011 [29].
2. Bisphenol A Analogues in Food
There is little information regarding the occurrence of bisphenol analogues (BPs) in foodstuffs. Liao and Kannan [30], in a study performed in the United States, determined the presence of BPA, BPF, and BPS (N = 267) in nine categories of foodstuffs and found that 75% of the samples contained BPs, with total concentrations ranging between below the limit of quantification (LOQ) and 1130 ng/g fresh weight (4.38 ng/g overall mean value). The highest overall mean concentration (sum of eight BPs) was detected in preserved and ready-to-eat foods. The highest BPF and bisphenol P (BPP) concentrations were 1130 ng/g and 237 ng/g, respectively, and were found in a sample of mustard and ginger. In contrast, BPs in beverages and fruits were found in concentrations of 0.341 ng/g and 0.698 ng/g, respectively. Higher levels of individual and total BPs were detected in canned food than in foods that came in plastic, glass, or paper containers.
Liao and Kannan [31], in a study performed in China, determined the presence of eight BPs (N = 289) in 13 categories of foodstuffs using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). The most frequently found BPs were BPA and BPF, which were detected at mean value concentrations of 4.94 ng/g and 2.50 ng/g fresh weight, respectively. The highest overall concentration (sum of eight BPs) was found in canned products (27.0 ng/g), followed by fish and seafood (16.5 ng/g), and beverages (15.6 ng/g). In contrast, the lowest overall concentration was found in milk and dairy products, cooking oils, and eggs (2–3 ng/g). Higher total concentration levels were detected in canned foodstuffs (56.9 ng/g) than in foods coming in glass (0.43 ng/g), paper (11.9 ng/g), or plastic (6.40 ng/g) containers.
Other studies have reported on the presence of BPA analogues in canned vegetables, fruits, and soft drinks [29][32] as well as in honey [33][34], fish [34], and mustard [35]. BPF has been found in mustard from white mustard seeds in mg kg−1 levels and is a natural reaction product formed during its preparation. Mustard is one of the most widely used condiments worldwide and, according to some authors, it is the main source of BPF in humans, in Europe, and probably worldwide [35]. In addition to BPA, bisphenol A diglycidyl ether (BADGE) and bisphenol F diglycidyl ether (BFDGE) have been detected in different milk samples from supermarkets and dairy farms [36], and BPF and BPS have been detected in dairy products, meat and meat products, vegetables, and cereals [30].
Although BPA is the most studied BP, BADGE and BFDGE have also been detected in drinking water as these BPs are commonly used in the epoxy coatings applied in drinking water distribution systems. BPs from the coatings may be exposed to chemical oxidants (disinfectants) that have the potential to form by-products with enhanced or reduced estrogenic activity than the parent compounds [37]. Exposure to free chlorine results in rapid BP degradation (half-lives of BPs are between <1 min to 35 min) under typical conditions [37].
There is an increasing amount of research linking long-term, low-level exposure to BPA in early life and adverse health effects in infants and fetuses [22]. Breast milk is the main source of energy for babies under six months and therefore it may be used as a proxy of the internal exposure levels in mothers and fetuses. Niu et al. (2017) [22] found BPA, BPF, BPS, and BPAF in breast milk samples, with BPA being the most abundant BP, followed by BPF. Recently, our research group has reported on the presence of BPB, BPS, BPE, and BPP in baby food samples [10].
3. Bisphenol A Analogues in Biological Samples and Their Hormonal Effects
Data on BPA analogue occurrence in human samples are scarce. Human uridine 5’-diphosphate-glucuronosyltransferases are able to rapidly metabolize BPA analogues into the corresponding BPA-glucuronide (UGTs) [38][39][40]. BPA-glucuronides are rapidly excreted in urine in rats and humans [41] and determination of urinary levels is considered a good biomarker of exposure to BPs [42].
Liao et al., (2012) [23] determined the total concentration of BPS in 315 urine samples, collected from the U.S. and different Asian countries, and detected BPS in 81% of the samples analyzed with variations between countries. BPS concentrations were between below the LOQ (0.02 ng/mL) and 21 ng/mL (geometric mean (GM): 0.168 ng/mL). The highest GM concentration of BPS was detected in samples from Japan (1.18 ng/mL).
Ye et al., (2015) [43] determined the concentration of BPA, BPS, BPF, and BPAF in 616 archived urine samples collected anonymously from volunteers during eight years. BPA was the most frequently detected chemical (74%–99%), whereas BPAF was detected in fewer than 3% of the samples. The detection frequencies were in the ranges of 74%–99% for BPA, 42%–88% for BPF, and 19%–74% for BPS. GM concentrations were in the ranges of 0.36–2.07 μg/L for BPA, 0.15–0.54 μg/L for BPF, and <0.1 to 0.25 μg/L for BPS. Although BPF concentrations were generally lower than other BPs, the 95th percentile concentration of BPF was similar or higher than that of BPA in most of the samples. The authors conclude that the significant changes in GM concentrations of BPA and BPS are consistent with a decline in BPA exposure and an increase in BPS exposure.
Lehmler et al., (2018) [44] investigated the association between the presence of BPA, BPF, and BPS in urine samples from adults (N = 1808) and children (N = 868) and different demographic and lifestyle variables; the BPA, BPS, and BPF levels were 95.7%, 89.4%, and 66.5%, respectively. In adults, the median levels of BPA were higher (1.24 μg/L) than BPF and BPS levels (0.35 and 0.37 μg/L, respectively). In children, the median BPA levels were also higher (1.25 μg/L) than BPF and BPS levels (0.32 and 0.29 μg/L, respectively). The study revealed associations between the different BPs and gender, race, income, physical activity, smoking, and alcohol intake.
Exposure of pregnant women to BPs is a particular concern, as these chemicals pass from mother to infant via breast milk, making this matrix a main target for exposure assessment of critical subpopulations. In this framework, Deceuninck et al. (2015) [45] investigated the presence of a large group of BPA analogues in breast milk samples (N = 30) and detected BPS in one sample at a 0.23 μg/kg concentration, whereas the rest of the BPA analogues investigated were not detected. In a study by Niu et al. (2017) [22] also on human breast milk from Chinese mothers, BPF, BPS, and BPAF were found, where BPA was still the most frequently compound detected, followed by BPF. This is the first study reporting on the presence of BPF and BPAF in human breast milk.
Available studies on BPF and BPS quantification in other biological matrices are still limited. The detection frequency of these compounds in human serum is relatively low [46]. BPS has been found in maternal and cord blood serum [47]. More recently, Song et al. (2019) [48] found a detection frequency of more than 65% for BPA, BPAF, and BPF in serum samples collected from residents living near recycling facilities where e-waste was being dismantled, with GM concentrations of 3.2, 0.0074, and 0.062 μg/mL, respectively.
The presence of BPA analogues in human biological samples suggests that it may have an effect on the body. The estrogen activity is the most widely studied effect of BPA, and its effects on other hormonal receptors have also been reported [40]. However, limited studies have reported that BPA analogues have endocrine-disrupting activities similar to those of BPA [17]. The estrogenicity of BPs was first reported in 1998 using an E-SCREEN assay in cultures of the human breast cancer cell line MCF7 [49]. Later on, in 2002, we demonstrated the effects of these chemicals on the expression of estrogen-controlled genes by measuring the induction of pS2 (mRNA and protein) and progesterone receptor as well as the expression of a luciferase reporter gene transfected into MVLN cells [27]. Subsequent studies have corroborated these findings [21]. It has also been reported that the estrogenic effect of some BPs is higher than that of BPA [50]. For example, BPS shows higher hormonal activity, which can likely be attributed to its strong polarity and the presence of a sulfonyl group [32][51] and its heat stability and resistance to light [10][45]. Moreover, BPS and BPF have been shown to be involved in breast cancer progression as much as BPA by inducing proliferation and migration of MCF-7 clonal cells [52]. Recently, Van Leeuwen et al., (2019) [53] reported that most BPA analogues in in vitro studies have similar or higher estrogenic activity than BPA as well as higher antiandrogenic properties. Other BPA analogues showed both antiestrogenic and antiandrogenic activity.
BPS and BPF are the most studied bisphenol analogues. A systematic review [28] that included 32 studies (25 in vitro and 7 in vivo) revealed that the potency of BPF and BPS was in the same order of magnitude as BPA and had similar hormonal effects. Additionally, the review showed that BPS and BPF had hormonal effects beyond those of BPA, such as changes in organ weights and enzyme expression levels. The authors concluded that BPS and BPF seemed to have similar potency and mechanisms of action to those of BPA, posing similar health effects. Other authors have also reported on the similarity between BPS and BPF and BPA in terms of their toxicological profiles, including metabolic, carcinogenic, and reproductive effects, as well as oxidative stress and DNA damage [18][28][54][55][56].
Some studies in animal models have also suggested the adverse reproductive effects secondary to exposure to BPA analogues, such as reduced sperm and oocyte quality and conversion of cholesterol into biologically active steroid hormones (steroidogenesis). These adverse effects depend on the duration of the exposure and the species investigated [57]. In this context, the effect of long-term BPF exposure on the reproductive neuroendocrine system in zebrafish has been recently demonstrated [58]. Similarly, in the same animal model, BPS was shown to decrease gonad weight and alter plasma estrogen and testosterone, as well as to reduce egg production and hatchability, with longer hatch periods, and increase embryo malformations. Shi et al., (2018) [59] showed that prenatal exposure to BPA analogues with physiologically relevant doses affects male reproductive functions probably due to a spermatogenic defect in the developing testis. Ullah et al. (2019) [60] demonstrated the impact of low-dose chronic exposure to BPB, BPF, and BPS on hypothalamo–pituitary–testicular activities in adult rats. The effect on female reproductive functions in mice after prenatal exposure to BPA analogues was recently demonstrated [61]. The authors concluded that prenatal exposure to bisphenols accelerated the onset of puberty, and the mice exhibited fertility problems, abnormal estrous cyclicity, and dysregulated expression of steroidogenic enzymes, especially with lower doses. Kolla et al., (2018) [62] compared the BPA and BPS exposure effect during the perinatal period on female mouse mammary gland development. The study revealed age- and dose-specific effects of BPS that were different from the effects of BPA. In addition, Zhou (2018) [63] evaluated low-concentration BPS toxicity using L1 larvae of the model animal Caenorhabditis elegans. Multiple indicators at the physiological, biochemical, and molecular levels were tested. Compared with the effects of BPA, the overall results showed that BPS was less noxious, suggesting that individual bisphenols may have unique effects. Other studies have also demonstrated the estrogenic, androgenic, and thyroidogenic activities of BPF and BPS [28][46].
Eladak et al., (2015) [64] developed a fetal testis assay to demonstrate that 10 nmol/L BPS and BPF can reduce the basal testosterone secretion by fetal human and mouse testes. Furthermore, more recently, Desdoits-Lethimonier et al., (2017) [65] showed that, using an ex vivo culture system, BPE, BPF, BPB, and BADGE exhibited antiandrogenic properties in adult human testes. In addition, in a study conducted on GH3 rat cell line, BPA, BPAF, BPB, BPF, BPS, and bisphenol Z (BPZ) have been found to alter the activity of the thyroid endocrine system, which seems to be increased by 17β-estradiol [66].
4. Obesogenic Effects of Bisphenol A Analogues
There is increasing evidence that a number of chemicals can interfere in hormonal metabolism and regulation of adipocyte function. This may result in imbalanced hormone levels, which can lead to obesity. These obesity-promoting compounds are known as “obesogens” [67].
It seems that genetic predisposition, unhealthy dietary habits as well as a sedentary lifestyle alone are not responsible for the global epidemic of overweightedness and obesity [67].
At a molecular level, another mechanism by which obesogens could lead to weight gain or obesity is through the activation of nuclear transcription factors, such as the peroxisome proliferator-activated receptors alpha, delta, and gamma (PPARα, PPAR-δ, and PPAR-γ) and steroid hormone receptors, that regulate adipocyte proliferation and differentiation and lipid metabolism, thereby influencing body composition. The transcription factors bind to response elements in the DNA-regulating specific patterns of gene expression [67]. Wassenaar et al., (2017) [68] conducted a systematic review of the literature and found a significant positive association between early-life exposure to BPA and fat weight and triglycerides.
BPA promotes adipogenesis, adipose tissue inflammation, and alteration of glucose and lipid metabolism [69]. BPA has the ability to bind to human and animal PPARγ, which could trigger obesogenic activities [70]. PPARγ is highly expressed in adipose tissue and regulates adipocyte development and the uptake of lipids by adipocytes. Obesogens can cause obesity through direct activation of PPARγ, but other mechanisms involve indirect activation of the receptor by increasing the PPARγ protein and making it available to promoters of genes in the adipogenic pathway [71]. Measurements of the effects of BPA exposure on obesity should consider not only the body mass index (BMI) but also the role of BPA in adipogenesis, lipid, and glucose dysregulation and the presence of adipose tissue inflammation, which in turn results from the increased secretion of proinflammatory compounds [69].
As with the hormonal effects of BPA substitutes, their obesogenic activity has not been well studied. However, the ability of BPS to promote accumulation of lipids and differentiation of human preadipocytes through the activation of a PPARγ pathway has been described [72]. Damage in the transcriptome of preadipocytes during their differentiation has been described in relation to long-term exposure to low doses of BPA or BPS and BPF [73]. As with BPA, BPS could induce differentiation of preadipocytes. Both BPA and BPS are weak activators of PPARγ and need this receptor to induce adipogenesis [74]. BPA and BPS can increase the differentiation of 3T3-L1 preadipocytes in mice in a dose-dependent manner. Moreover, BPS has shown stronger adipogenic activity than BPA [74].
Ivry Del Moral et al., (2016) [75] showed that BPS can increase the harmful effects of a high-fat diet and induce changes in the postprandial lipid metabolism, increasing fat accumulation in the adipose tissue.
Recently, Charisiadis et al., (2018) [76] investigated the relation between obesity and the presence of BPA and BPF in hypothalamic and white matter postmortem material from 12 pairs of obese (BMI >30 kg/m2) and normal-weight individuals. A significant association (p < 0.05) between BPA and BPF concentration and obesity was found, except for BPF in white matter samples.
In another study by Liu et al. (2017) [47] conducted in 1521 adults (≥20 years) who participated in the National Health and Nutrition Examination Survey 2013–2014, significant associations were found between BPA exposure and general and abdominal obesity, whereas no significant associations were found for BPF or BPS, at the current exposure level. However, the authors suggested continued biomonitoring of these BPA substitutes.
In addition, a recent study by Liu et al., (2019) [77] showed for the first time that exposure to BPF was positively associated with a higher risk of obesity in children and adolescents. This association was mainly found in boys, suggesting a potential gender influence.
References
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258.
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The in Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2018, 6, 2.
- Anifandis, G.; Katsanaki, K.; Lagodonti, G.; Messini, C.; Simopoulou, M.; Dafopoulos, K.; Daponte, A. The Effect of Glyphosate on Human Sperm Motility and Sperm DNA Fragmentation. Int. J. Environ. Res. Public Health 2018, 15, 1117.
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89.
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Stir Bar Sorptive Extraction Coupled to Gas Chromatography-Mass Spectrometry for the Determination of Bisphenols in Canned Beverages and Filling Liquids of Canned Vegetables. J. Chromatogr. A 2012, 1247, 146–153.
- García, J.A.; Gallego, C.; Font, G. Toxicidad del Bisfenol A: Revisión. Rev. Toxicol. 2015, 32, 144–160.
- Chen, M.Y.; Ike, M.; Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol A and other bisphenols. Environ. Toxicol. Int. J. 2002, 17, 80–86.
- Regueiro, J.; Breidbach, A.; Wenzl, T. Derivatization of bisphenol A and its analogues with pyridine-3-sulfonyl chloride: Multivariate optimization and fragmentation patterns by liquid chromatography/Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 1473–1484.
- Regueiro, J.; Wenzl, T. Determination of bisphenols in beverages by mixed-mode solid-phase extraction and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1422, 230–238.
- García-Córcoles, M.T.; Cipa, M.; Rivas, A.; Olea-Serrano, F.; Vílchez, A.J.; Zafra-Gómez, A. Determination of Bisphenols with Estrogenic Activity in Plastic Packaged Baby Food Samples Using Solid-Liquid Extraction and Clean-up with Dispersive Sorbents Followed by Gas Chromatography Tandem Mass Spectrometry. Talanta 2018, 178, 441–448.
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155.
- Barroso, J.M. European Commission Directive 2011/8/EU of 28 January 2011 amending directive 2002/72/EC as regards the restriction of use of bisphenol A in plastic infant feeding bottles. Off. J. Eur. Union 2011, 26, 11–14.
- European Commission. Commission Regulation (EU) 2018/213 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation No 10/2011 as regards the use of that substance in plastic food. 2018. Available online: http://data.europa.eu/eli/reg/2018/213/oj (accessed on 31 August 2019).
- French Republic, Regulation No. 1442/2012 of 24 December 2012 Aiming at Banning the Manufacture, Import, Export and Commercialisation of All Forms of Food Packaging Containing Bisphenol A. Off. J. French Republic 2012. Available online: https://www.legifrance.gouv.fr/jo_pdf.do?numJO=0&dateJO=20121226&numTexte=2&pageDebut=20395&pageFin=20396 (accessed on 31 August 2019).
- Food and Drug Administration (FDA). Indirect Food Additives: Polymers; Federal Register: Rockville, MD, USA, 2012; Volume 77, pp. 41899–41902.
- Food and Drug Administration (FDA). Indirect Food Additives: Adhesives and Components of Coatings; Federal Register: Rockville, MD, USA, 2013; Volume 78, pp. 41840–41843.
- Liao, C.; Liu, F.; Kannan, K.; Bisphenol, S. A new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues. Environ. Sci. Technol. 2012, 46, 6515–6522.
- Gallo, P.; Pisciottano, I.D.M.; Esposito, F.; Fasano, E.; Scognamiglio, G.; Mita, G.D.; Cirillo, T. Determination of BPA, BPB, BPF, BADGE and BFDGE in Canned Energy Drinks by Molecularly Imprinted Polymer Cleaning up and UPLC with Fluorescence Detection. Food Chem. 2017, 220, 406–412.
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259.
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol. Lett. 2012, 211, 201–209.
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity: A review. Environ. Sci. Technol. 2012, 50, 5438–5453.
- Niu, Y.; Wang, B.; Zhao, Y.; Zhang, J.; Shao, B. Highly sensitive and high-throughput method for the analysis of bisphenol analogues and their halogenated derivatives in breast milk. J. Agric. Food Chem. 2017, 65, 10452–10463.
- Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.B.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866.
- Kojima, H.; Takeuchi, S.; Sanoh, S.; Okuda, K.; Kitamura, S.; Uramaru, N.; Sugihara, K.; Yoshinari, K. Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 2019, 413, 48–55.
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.H.; Svoboda, D.; Auerbach, S.S.; et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235.
- Punt, A.; Aartse, A.; Bovee, T.F.H.; Gerssen, A.; Van Leeuwen, S.P.J. Hoogenboom RLAP, Peijnenburg AACM. Quantitative in vitro-to-in vivo extrapolation (QIVIVE) of estrogenic and anti-androgenic potencies of BPA and BADGE analogues. Arch. Toxicol. 2019, 93, 1941–1953.
- Rivas, A.; Lacroix, M.; Olea-Serrano, F.; Laos, I.; Leclercq, G.; Olea, N. Estrogenic effect of a series of bisphenol analogues on gene and protein expression in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002, 82, 45–53.
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650.
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Fast liquid chromatographytandem mass spectrometry for the analysis of bisphenol A-diglycidyl ether, bisphenol F-diglycidyl ether and their derivatives in canned food and beverages. J. Chromatogr. A 1218, 2011, 1603–1610.
- Liao, C.; Kannan, K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the united states and their implications for human exposure. J. Agric. Food Chem. 2013, 61, 4655–4662.
- Liao, A.; Kannan, K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 319–329.
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Analysis of bisphenols in soft drinks by on-line solid phase extraction fast liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2011, 683, 227–233.
- Cesen, M.; Lambropoulou, D.; Laimou-Geraniou, M.; Kosjek, T.; Blanznik, U.; Heath, D.; Heath, E. Determination of Bisphenols and Related Compounds in Honey and Their Migration from Selected Food Contact Materials. J. Agric. Food. Chem. 2016, 64, 8866–8875.
- Sadeghi, M.; Nematifar, Z.; Fattahi, N.; Pirsaheb, M.; Shamsipur, M. Determination of Bisphenol A in Food and Environmental Samples Using Combined Solid-Phase Extraction-Dispersive Liquid-Liquid Microextraction with Solidification of Floating Organic Drop Followed by HPLC. Food Anal. Methods 2016, 9, 1814–1824.
- Zoller, O.; Brüschweiler, B.J.; Magnin, R.; Reinhard, H.; Rhyn, P.; Rupp, H.; Zeltner, S.; Felleisen, R. Natural occurrence of bisphenol F in mustard. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 137–146.
- Xionga, L.; Yana, P.; Chua, M.; Gaoa, Y.Q.; Lia, W.H.; Yanga, X.L. A rapid and simple HPLC-FLD screening method with QuEChERS as the sample treatment for the simultaneous monitoring of nine bisphenols in milk. Food Chem. 2018, 244, 371–377.
- Lane, R.F.; Adams, C.D.; Randtke, S.J.; Carter, R.E. Chlorination and chloramination of bisphenol A, bisphenol F, and bisphenol A diglycidyl ether in drinking water. Water Res. 2015, 79, 68–78.
- Pottenger, L.H.; Domoradzki, J.Y.; Markham, D.A.; Hansen, S.C.; Cagen, S.Z.; Waechter, J.M., Jr. The Relative Bioavailability and Metabolism of Bisphenol A in Rats Is Dependent upon the Route of Administration. Toxicol. Sci. 2000, 54, 3–18.
- Hanioka, N.; Naito, T.; Narimatsu, S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 2008, 74, 33–36.
- Gramec-Skledar, D.; Peterlin-Mašič, L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199.
- Taylor, J.A.; vom Saal, F.S.; Welshons, W.V.; Drury, B.; Rottinghaus, G.; Hunt, P.A.; Toutain, P.-L.; Laffont, C.M.; VandeVoort, C.A. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environ. Health Perspect. 2011, 119, 422–430.
- Koch, H.M.; Kolossa-Gehring, M.; Schröter-Kermani, C.; Angerer, J.; Brüning, T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 610–616.
- Ye, X.; Wong, L.-Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of US adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839.
- Lehmler, H.J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013-2014. ACS Omega 2018, 3, 6523–6532.
- Deceuninck, Y.; Bichon, E.; Marchand, P.; Boquien, C.Y.; Legrand, A.; Boscher, C.; Antignac, J.P.; Le Bizec, B. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2485–2497.
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int. 2015, 83, 107–115.
- Liu, B.; Lehmler, H.J.; Sun, Y.; Xu, G.; Liu, Y.; Zong, G.; Sun, Q.; Hu, F.B.; Wallace, R.B.; Bao, W. Bisphenol A substitutes and obesity in US adults: Analysis of a population-based, cross-sectional study. Lancet Planet. Health 2017, 1, e114–e122.
- Song, S.; Duan, Y.; Zhang, T.; Zhang, B.; Zhao, Z.; Bai, X.; Xie, L.; He, Y.; Ouyang, J.P.; Huang, X.; et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose. Ecotoxicol. Environ. Saf. 2019, 106, 822–828.
- Perez, P.; Pulgar, R.; Olea-Serrano, F.; Villalobos, M.; Rivas, A.; Metzler, M.; Pedraza, V.; Olea, N. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ. Health Perspect. 1998, 106, 167–174.
- Cao, H.; Wang, F.; Liang, Y.; Wang, H.; Zhang, A.; Song, M. Experimental and computational insights on the recognition mechanism between the estrogen receptor α with bisphenol compounds. Arch. Toxicol. 2017, 91, 3897–3912.
- Caballero-Casero, N.; Lunar, L.; Rubio, S. Analytical methods for the determination of mixtures of bisphenols and derivatives in human and environmental exposure sources and biological fluids. A review. Anal. Chim. Acta 2016, 908, 22–53.
- Kim, J.; Choi, H.; Lee, H.; Lee, G.; Hwang, K.; Choi, K. Effects of bisphenol compounds on the growth and epithelial mesenchymal transition of MCF-7 CV human breast cancer cells. J. Biomed. Res. 2017, 31, 358–369.
- Van Leeuwen, S.P.; Bovee, T.F.; Awchi, M.; Klijnstra, M.D.; Hamers, A.R.; Hoogenboom, R.L.; Portier, L.; Gerssen, A. BPA, BADGE and analogues: A new multi-analyte LC-ESI-MS/MS method for their determination and their in vitro (anti)estrogenic and (anti)androgenic properties. Chemosphere 2019, 221, 246–253.
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; Van Vugt-Lussenburg, B.M.A.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. 2014, 139, 35–47.
- Roelofs, M.J.E.; van den Berg, M.; Bovee, T.F.H.; Piersma, A.H.; Van Duursen, M.B.M. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology 2015, 329, 10–20.
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98.
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123.
- Qui, W.; Fang, M.; Liu, C.; Zheng, C.; Chen, B.; Wang, K.J. In vivo actions of bisphenol F on the reproductive neuroendocrine system after long-term exposure in zebrafish. Sci. Total Environ. 2019, 15, 995–1002.
- Shi, M.; Sekulovski, N.; MacLean, J.A., 2nd; Hayashi, K. Prenatal Exposure to Bisphenol A Analogues on Male Reproductive Functions in Mice. Toxicol Sci. 2018, 163, 620–631.
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Turi, N.; Ullah, W.; Siddiqui, M.F.; Zakria, M.; Lodhi, K.Z.; Khan, M.M. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 2018, 121, 24–36.
- Shi, M.; Sekulovski, N.; MacLean, J.A.; Whorton, A.; Hayashi, K. Prenatal Exposure to Bisphenol A Analogues on Female Reproductive Functions in Mice. Toxicol. Sci. 2019, 168, 561–571.
- Kolla, S.; Morcos, M.; Martin, B.; Vandenberg, L.N. Low dose bisphenol S or ethinyl estradiol exposures during the perinatal period alter female mouse mammary gland development. Reprod. Toxicol. 2018, 78, 50–59.
- Zhou, D. Ecotoxicity of bisphenol S to Caenorhabditis elegans by prolonged exposure in comparison with bisphenol A. Environ. Toxicol. Chem. 2018, 37, 2560–2565.
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21.
- Desdoits-Lethimonier, C.; Lesné, L.; Gaudriault, P.; Zalko, D.; Antignac, J.P.; Deceuninck, Y.; Platel, C.; Dejucq-Rainsford, N.; Mazaud-Guittot, S.; Jégou, B. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum. Reprod. 2017, 32, 1465–1473.
- Lee, J.; Kim, S.; Choi, K.; Ji, K. Effects of bisphenol analogs on thyroid endocrine system and possible interaction with 17β-estradiol using GH3 cells. Toxicol. Vitr. 2018, 53, 107–113.
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27.
- Wassenaar, N.H.; Trasando, L.; Legler, J. Systematic Review and Meta-Analysis of Early-Life Exposure to Bisphenol A and Obesity-Related Outcomes in Rodents. Environ. Health Perspect. 2017, 125, 106001.
- Legeay, S.; Faure, S. Is bisphenol A an environmental obesogen? Fund. Clin. Pharmacol. 2017, 31, 594–609.
- Riu, A.; Grimaldi, M.; Le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogues of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232.
- Zheng, S.; Shi, J.; Zhang, J.; Yang, Y.; Hu, J.; Shaon, B. Identification of the disinfection byproducts of bisphenol S and the disrupting effect on peroxisome proliferator-activated receptor gamma (PPARg) induced by chlorination. Water Res. 2018, 132, 167–176.
- Boucher, J.G.; Ahmed, S.; Atlas, E. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology 2016, 157, 1397–1407.
- Verbanck, M.; Canouil, M.; Leloire, A.; Dhennin, V.; Coumoul, X.; Yengo, L.; Froguel, P.; Poulain-Godefroy, O. Low-dose exposure to bisphenols A, F and S of human primary adipocyte impacts coding and non-coding RNA profiles. PLoS ONE 2017, 12, e0179583.
- Ahmed, S.; Atlas, E. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferatoractivated receptor gamma activation. Int. J. Obes. 2016, 40, 1566–1573.
- Ivry Del Moral, L.; Le Corre, L.; Poirier, H.; Niot, I.; Truntzer, T.; Merlin, J.F.; Rouimi, P.; Besnard, P.; Rahmani, R.; Chagnon, M.C. Obesogen effects after perinatal exposure of 4,4’-sulfonyldiphenol (Bisphenol S) in C57BL/6 mice. Toxicology 2016, 357, 11–20.
- Charisiadis, P.; Andrianou, X.D.; van der Meer, T.P.; den Dunnen, W.F.A.; Swaab, D.F.; Wolffenbuttel, B.H.R.; Makris, K.C.; van Vliet-Ostaptchouk, J.V. Possible obesogenic effects of bisphenols accumulation in the human brain. Sci. Rep. 2018, 8, 1–10.
- Liu, B.; Lehmler, H.-J.; Sun, Y.; Xu, G.; Sun, Q.; Snetselaar, L.G.; Wallace, R.B.; Bao, W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab. J. 2019, 43, 59.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
4 times
(View History)
Update Date:
06 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




