
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alissara Reungsang | + 13048 word(s) | 13048 | 2021-08-23 09:58:10 | | | |
| 2 | Alissara Reungsang | Meta information modification | 13048 | 2021-09-04 14:51:32 | | | | |
| 3 | Alissara Reungsang | -11704 word(s) | 1344 | 2021-09-10 07:51:54 | | | | |
| 4 | Catherine Yang | Meta information modification | 1344 | 2021-09-28 08:09:22 | | |
Video Upload Options
Volatile fatty acids (VFAs) are low-molecular-weight carboxylic acids containing two to six carbon atoms that are produced as the final product of acidogenic fermentation in anaerobic digestion process.
1. Introduction
VFA production by biological routes from organic waste is gaining attention because of its economic and environmental advantages. VFAs, including acetic acid, propionic acid, butyric acid, caproic acid, and valeric acid, are produced as the final products of acidogenic fermentation in the anaerobic digestion process. These compounds are reported to be the most critical precursors in the biorefinery concept. They can be directly or indirectly used as carbon sources for biological nutrient removal from wastewater [1] or as precursors to synthesize high-value-added products, such as bioplastics [2] and biodiesel [3].
Although several studies have investigated the process and operational parameters of VFAs production, the process cannot be easily scaled up to the pilot-scale because of limitations in the recovery of the produced VFAs [4]. The challenges in VFA recovery include: 1) processing cost, which accounts for approximately 30–40% of the total production cost, 2) the mixture complexity of fermentation broth, and 3) VFAs with low concentration in the fermented stream [1][5]. Furthermore, the presence of suspended solids including cells and inorganic precipitates in real fermentation broth might hinder mass transfer coefficients of the VFA recovery step. Various techniques have been applied for VFA recovery, including liquid–liquid extraction [6], adsorption [7][8], membrane contactors [9], membrane reactors [10][11], electrodialysis [12] and membrane pervaporation [13]. Among them, membrane-based recovery appears to be a more promising technology than other techniques because of its economic and environmental potential [1][4][14].
2. Membrane-Based VFA Recovery
VFA recovery can be broadly classified into non-membrane-based and membrane-based recovery. Previous studies have provided a complete explanation of the various strategies for recovery of VFAs by conventional non-membrane-based methods, including solvent extraction, distillation, chemical precipitation, adsorption, and ion exchange [4][14][15]. However, current researchers have focused on applying membrane technology to achieve effective VFA recovery strategies because it could reduce the number of recovery steps while shortening the residence time, resulting in a more economical process [14][16]. Table 1 summarizes the advantages and disadvantages of membrane-based techniques for VFA recovery from acidogenic effluents.
Table 1. Membrane-based VFA recovery techniques.
|
Processes |
Principle |
Advantages |
Disadvantages |
Ref. |
|
Membrane contactor |
Hydrophobic membrane is used to separate two aqua phases. The volatile species (e.g., VFAs) from the feed side would transfer into the permeate side until the partial pressure or concentration gradient between the two sides are in equilibrium under isothermal conditions. |
· VFAs selectivity can be improved by filling specific extractants. · Can be directly connected to a reactor |
· The number of carbon atoms limits recovery efficiency. · High ions and solid content negatively affect recovery. · Extractant is needed |
|
|
Electrodialysis |
The positively charged ions (cations) in the solution move toward the cathode. Likewise, the negatively charged ions (anions) move toward the anode. The cations pass through the cation-exchange membrane into the concentrate compartments, but the anion-exchange membrane retains them. Simultaneously, the anions pass through the anion membrane into the concentrate compartments, but the cation-exchange membrane retains them. The overall result in concentrating of VFAs in concentrate compartments while ions in dilute compartments are depleted. |
· No need for high pressure · Directly connected to side stream or in situ process is possible. · High VFA recovery rate |
· Not selective to VFAs alone, leading to loss of nutrients, such as NO3- and PO43- · High energy demand · Back diffusion · The cost associated with membrane maintenance and replacement. |
|
|
Anaerobic membrane bioreactor |
Bioreactor combined with pressure-driven microfiltration system to separate the VFA solution from the mixture (fermentation broth). VFA solution passes through a semipermeable membrane, whereas the solids, impurities, microorganisms that are too large to pass through membrane pores are retained on the membrane surface. |
· Can be operated under high-solid loading and high-medium viscosity. · Cell retention with minimal washout rate · In line VFA recovery · Easy to scale up |
· Control of membrane fouling · Biofilm formation · Not selective for VFAs |
|
|
Membrane pervaporation |
Non-porous membrane was used to separate between VFA feed solution and vapor-permeate phase. VFAs are transferred across the membrane into a vapor-permeate phase by the difference in chemical potential gradient established by the difference in the partial pressure. |
· High concentration of recovered VFAs · No adverse effects on microbial growth · Low capital cost |
· Intensive energy input · Control of membrane fouling · Control of pH of the feed · Impurities hinder VFA recovery |
2.1 Membrane Contactor
The membrane contactor uses a hydrophobic porous membrane to separate the two aqueous phases on opposite sides of the membrane, avoiding mixing (Figure 1) [14][20]. The commonly used polymers in membrane contactors, selected based on their properties, such as mechanical stability, chemical resistance, and hydrophilic or hydrophobic characteristics, are polypropylene (PP), polyvinylidene fluoride (PVDF), and polytetrafluoroethylene (PTFE) [21]. The transmembrane pressure is determined by the partial pressure or concentration difference between the two sides of the membrane. In most membrane contactor processes, the driving force is created by the pH gradient between the two sides of the membrane.
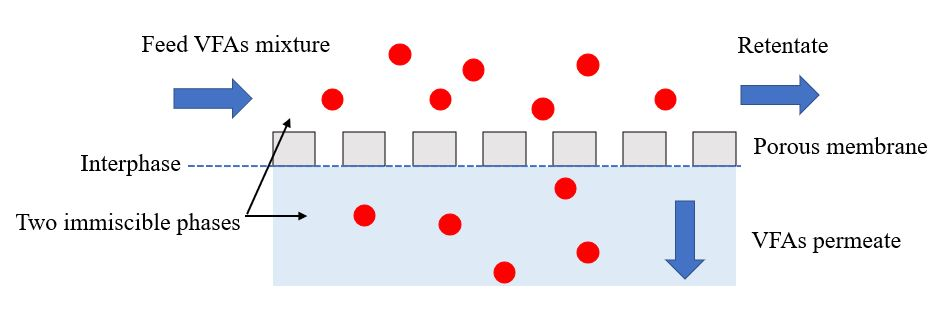
Figure 1. Operating principle of VFA recovery by membrane contactor.
2.2 Electrodialysis
Electrodialysis is a membrane separation process used to transport ionic solutes from one solution to another through anion and cation-exchange membranes separated by a spacer gasket in an alternating pattern forming individual cells between two electrodes [4], as illustrated in Figure 2.
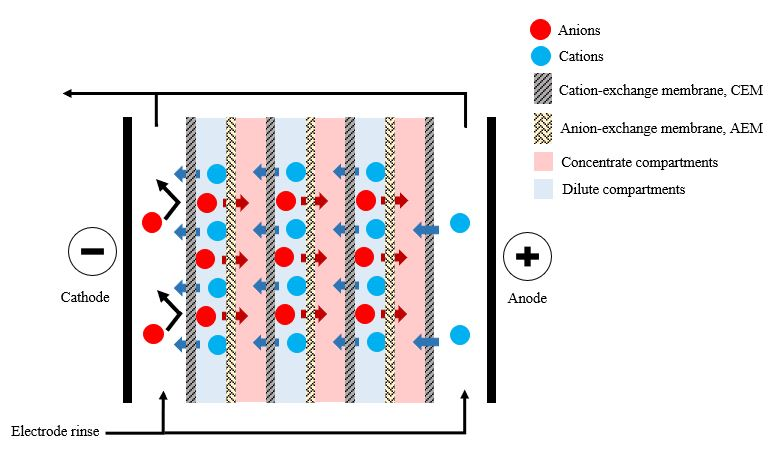
Figure 2. Schematic representation of electrodialysis.
The VFA-rich fermentation broth was transferred through the microfiltration system before feeding it into the electrodialysis stack to prevent solid particles from interfering with electrodialysis operation and to reducing the fouling tendency of the downstream process [15]. Previous studies found that, even though electrodialysis concentrated approximately 90% of the VFAs in the permeate, each acid behaved differently. The transport efficiency of small molecular weight acids was greater than that of larger molecular weight acids [12][22].
2.3 Anaerobic Membrane Bioreactor
Anaerobic membrane technology is a hybrid system in which membranes are combined with an anaerobic bioreactor (Figure 3) [23]. Anaerobic membrane bioreactors have emerged as a promising alternative to VFA recovery technologies because of their low sludge generation and energy requirements. Moreover, it has the benefit of reducing the operating space and number of operational units [23]. Integrating a membrane recovery system with an acidogenic fermentation bioreactor would decrease cell washout from the bioreactor and the inhibition effect of the acids, thereby improving biodegradation [24].
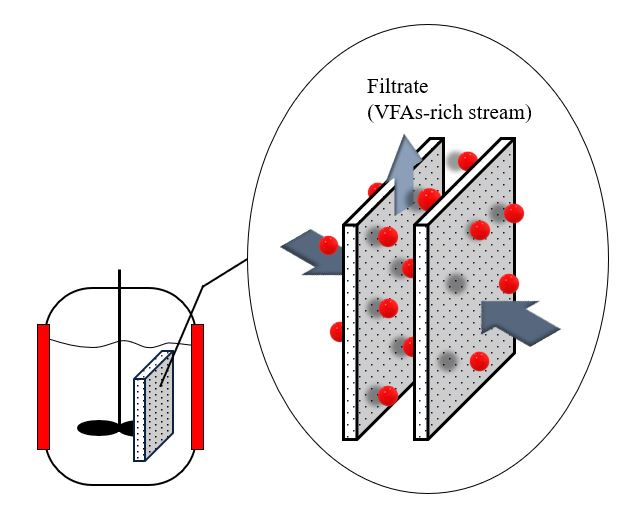
Figure 3. Anaerobic membrane bioreactor.
Long-term VFA production and recovery from acidogenic fermentation of cow manure were investigated by Jomnonkhaow et al. (2020) [10]. The authors observed that the maximum VFA yield of 0.41 g-VFAs/g-VS was obtained from heat-treated cow manure without pH control or inoculum addition. Furthermore, anaerobic membrane bioreactors can be operated at very high amounts of TSS ranging from 15 to 55 g-total suspended solid (TSS)/L by employing a routine reactor draining, backwashing, gas sparging, and chemical membrane cleaning to control the amount of TSS in the reactor. Consistent with the study by Parchami et al. (2020) [1], they observed that even though the anaerobic membrane bioreactor was operated at a maximum suspended solid loading of 32 g/L, minimal filtration flux deterioration was observed. Other studies have shown that anaerobic membrane bioreactors have the potential to operate at high organic loading with a complex medium for a long period [24][25].
2.3 Membrane Pervaporation
The membrane pervaporation process relies on diffusing different components in the feed mixture through a dense membrane [14][21]. Pervaporation membranes can also be broadly categorized into two groups based on the target molecule to be recovered: hydrophilic pervaporation membranes ensure rapid water transport, allowing for effective dehydration, whereas organophilic pervaporation membranes provide better transport of organic compounds, making them suitable for VFA recovery [20]. The pervaporation process is also more suitable for separating VFAs from water (azeotropic mixtures) compared with other separation methods [6]. Additionally, membrane pervaporation has no adverse effects on microorganisms in the fermentation broth and can be directly integrated with an acidogenic bioreactor to continuously remove inhibitory products [5]. The driving force in membrane pervaporation is the chemical potential gradient across the membrane, which can be created by applying a vacuum or gas purge on the permeate side to keep the permeate vapor pressure lower than the partial pressure of the feed liquid [14][21]. The membrane controls the selective transport of species from the feed-liquid mixture to the vapor-permeate (Figure 4).
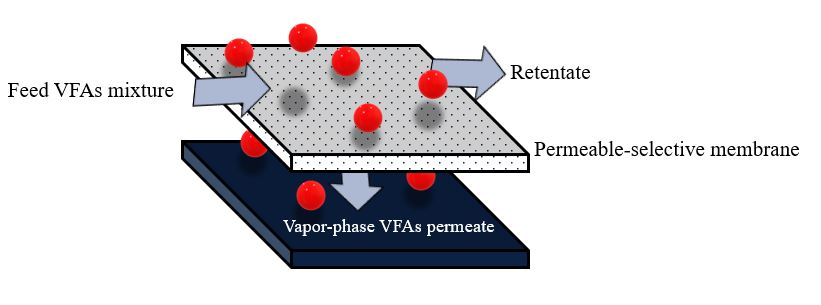
Figure 4. Schematic diagram of membrane pervaporation.
References
- Parchami, Mohsen; Steven, Wainaina; Mahboubi, Amir; I’Ons, David; Taherzadeh J., Mohammad; MBR-Assisted VFAs Production from Excess Sewage Sludge and Food Waste Slurry for Sustainable Wastewater Treatment. Applied Sciences 2020, 10, 2921, https://doi.org/10.3390/app10082921.
- Al Battashi, Huda; Al‑Kindi, Shatha; Kumar Gupta, Vijai; Sivakumar Nallusamy; Polyhydroxyalkanoate (PHA) Production Using Volatile Fatty Acids Derived from the Anaerobic Digestion of Waste Paper. Journal of Polymers and the Environment 2020, 29, 250-259, https://doi.org/10.1007/s10924-020-01870-0.
- Patel, Alok; Mahboubi, Amir; Horváth Sárvári, Ilona; Taherzadeh J., Mohammad; Rova, Ulrika; Christakopoulos, Paul; Matsakas, Leonidas; Volatile Fatty Acids (VFAs) Generated by Anaerobic Digestion Serve as Feedstock for Freshwater and Marine Oleaginous Microorganisms to Produce Biodiesel and Added-Value Compounds. Frontiers in Microbiology 2021, 12, 614612, doi:10.3389/fmicb.2021.614612.
- Atasoy, Merve; Owusu-Agyeman, Isaac; Plaza, Ezbieta; Cetecioglu, Zeynep; Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresource Technology 2018, 268, 773-786, https://doi.org/10.1016/j.biortech.2018.07.042.
- Yesil, Hatice; Taner, Hatice; Nigiz Ugur, Filiz; Hilmioglu, Nilufer; Tugtas Evren, Adile; Pervaporative Separation of Mixed Volatile Fatty Acids: A Study Towards Integrated VFA Production and Separation. Waste and Biomass Valorization 2020, 11, 1737–1753, https://doi.org/10.1007/s12649-018-0504-6.
- Reyhanitash, Ehsan; Fufachev, Egor; van Munster D., Kaspar; van Beek B. M., Michael; Sprakel M. J., Lisette; Edelijn N., Carmen; Weckhuysen M., Bert; Kersten R. A., Sascha; Bruijnincx C. A., Pieter; Schuur, Boelo; et al. Recovery and conversion of acetic acid from a phosphonium phosphinate ionic liquid to enable valorization of fermented wastewater. Green Chemistry 2019, 21, 2023-2034, https://doi.org/10.1039/C9GC00725C.
- Eregowd, Tejaswini; Rene R., Eldon; Rintala Jukka; Lens N. L., Piet; Volatile fatty acid adsorption on anion exchange resins: kinetics and selective recovery of acetic acid. Separation Science and Technology 2020, 55, 1449-1461, https://doi.org/10.1080/01496395.2019.1600553.
- Talebi, Amir; Razali Syafikah, Yasmin; Ismail, Norli; Rafatullah, Mohd; Tajarudin Azan, Husnul; Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Science of The Total Environment 2020, 707, 134533, https://doi.org/10.1016/j.scitotenv.2019.134533.
- Aydin, Senem; Yesil, Hatice; Tugtas Evren, Adile; Recovery of mixed volatile fatty acids from anaerobically fermented organic wastes by vapor permeation membrane contactors. Bioresource Technology 2018, 250, 548-555, https://doi.org/10.1016/j.biortech.2017.11.061.
- Jomnonkhaow, Umarin; Uwineza, Clarisse; Mahboubi, Amir; Wainaina, Steven; Reungsang, Alissara; Taherzadeh J., Mohammad; Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresource Technology 2021, 321, 124456, https://doi.org/10.1016/j.biortech.2020.124456.
- Lukitawesa; Eryildiz, Bahriye; Mahboubi, Amir; Millati, Ria; Mohammad T., aherzadehJ.; Semi-continuous production of volatile fatty acids from citrus waste using membrane bioreactors. Innovative Food Science & Emerging Technologies 2021, 67, 102545, https://doi.org/10.1016/j.ifset.2020.102545.
- Hassan K., Gamal; Massanet-Nicolau, Jaime; Dinsdale, Richard; Jones, Rhys Jon; M.M., Abo-Aly; Fatma, A.El-Gohary; Guwy, Alan; A novel method for increasing biohydrogen production from food waste using electrodialysis. International Journal of Hydrogen Energy 2019, 44, 14715-14720, https://doi.org/10.1016/j.ijhydene.2019.04.176.
- Hatice Yesil; Hatice Taner; Filiz Ugur Nigiz; Nilufer Hilmioglu; A. Evren Tugtas; Pervaporative Separation of Mixed Volatile Fatty Acids: A Study Towards Integrated VFA Production and Separation. Waste and Biomass Valorization 2018, 11, 1737-1753, 10.1007/s12649-018-0504-6.
- Sadegh Aghapour Aktij; Alireza Zirehpour; Arash Mollahosseini; Mohammad J. Taherzadeh; Alberto Tiraferri; Ahmad Rahimpour; Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. Journal of Industrial and Engineering Chemistry 2019, 81, 24-40, 10.1016/j.jiec.2019.09.009.
- Fan Lü; Zhijie Wang; Hua Zhang; Liming Shao; Pinjing He; Anaerobic digestion of organic waste: Recovery of value-added and inhibitory compounds from liquid fraction of digestate. Bioresource Technology 2021, 333, 125196, 10.1016/j.biortech.2021.125196.
- Doga Arslan; Y. Zhang; K.J.J. Steinbusch; L. Diels; Hubertus Hamelers; C.J.N. Buisman; H. De Wever; In-situ carboxylate recovery and simultaneous pH control with tailor-configured bipolar membrane electrodialysis during continuous mixed culture fermentation. Separation and Purification Technology 2017, 175, 27-35, 10.1016/j.seppur.2016.11.032.
- Rhiannon Chalmers Brown; Romain Tuffou; Jaime Massanet Nicolau; Richard Dinsdale; Alan Guwy; Overcoming nutrient loss during volatile fatty acid recovery from fermentation media by addition of electrodialysis to a polytetrafluoroethylene membrane stack. Bioresource Technology 2019, 301, 122543, 10.1016/j.biortech.2019.122543.
- Xin-Rong Pan; Wen-Wei Li; Liang Huang; Hou-Qi Liu; Yun-Kun Wang; Yi-Kun Geng; Kwan Sing Paul Lam; Han-Qing Yu; Recovery of high-concentration volatile fatty acids from wastewater using an acidogenesis-electrodialysis integrated system. Bioresource Technology 2018, 260, 61-67, 10.1016/j.biortech.2018.03.083.
- Santosh K. Choudhari; Federico Cerrone; Trevor Woods; Kieran Joyce; Vincent O'Flaherty; Kevin O’ Connor; Ramesh Babu; Pervaporation separation of butyric acid from aqueous and anaerobic digestion (AD) solutions using PEBA based composite membranes. Journal of Industrial and Engineering Chemistry 2015, 23, 163-170, 10.1016/j.jiec.2014.08.010.
- Zhu, Xiaobo; Leininger, Aaron; Jassby, David; Tsesmetzis, Nicolas; Ren Jason, Zhiyong; Will Membranes Break Barriers on Volatile Fatty Acid Recovery from Anaerobic Digestion?. ACS ES&T Engineering 2021, 1, 141–153, https://doi.org/10.1021/acsestengg.0c00081.
- Basile, Angelo; Galiano, F.; Santoro S.,Figoli Alberto . Pervaporation and membrane contactors ; Basile, Angelo; De Falco, Marcello; Centi, Gabriele; Iaquaniello Gaetano, Eds.; John Wiley & Sons, Inc.: New Jersey, 2016; pp. 280-312.
- Bing Tao; Pearl Passanha; Philemon Kumi; Victoria Wilson; Dean Jones; Sandra Esteves; Recovery and concentration of thermally hydrolysed waste activated sludge derived volatile fatty acids and nutrients by microfiltration, electrodialysis and struvite precipitation for polyhydroxyalkanoates production. Chemical Engineering Journal 2016, 295, 11-19, 10.1016/j.cej.2016.03.036.
- Muhammad Maaz; Muhammad Yasin; Muhammad Aslam; Gopalakrishnan Kumar; A.E. Atabani; Mubbsher Idrees; Fatima Anjum; Farrukh Jamil; Rizwan Ahmad; Asim Laeeq Khan; et al.Geoffroy LesageMarc HeranJeonghwan Kim Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresource Technology 2019, 283, 358-372, 10.1016/j.biortech.2019.03.061.
- Steven Wainaina; Lukitawesa; Mukesh Kumar Awasthi; Mohammad Taherzadeh; Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437-458, 10.1080/21655979.2019.1673937.
- Mohd Atiqueuzzaman Khan; Huu Hao Ngo; Wenshan Guo; Yiwen Liu; Long Nghiem; Soon Woong Chang; Dinh Duc Nguyen; Shicheng Zhang; Gang Luo; Hui Jia; et al. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresource Technology 2018, 271, 100-108, 10.1016/j.biortech.2018.09.075.




