
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brandon Lee | + 1935 word(s) | 1935 | 2021-09-01 10:20:04 | | | |
| 2 | Jessie Wu | Meta information modification | 1935 | 2021-09-03 11:15:19 | | | | |
| 3 | Jessie Wu | + 1 word(s) | 1936 | 2021-09-03 11:17:31 | | | | |
| 4 | Jessie Wu | -1 word(s) | 1935 | 2021-09-03 11:33:14 | | | | |
| 5 | Brandon Lee | Meta information modification | 1935 | 2021-09-06 19:04:01 | | |
Video Upload Options
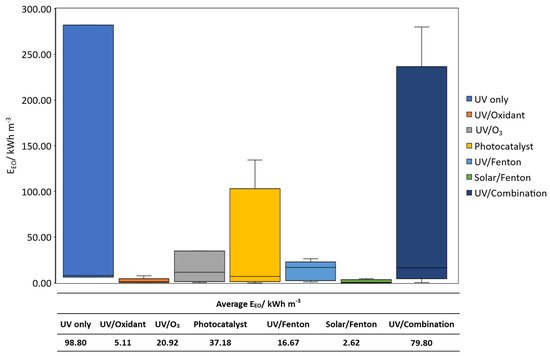
Contaminants of emerging concern (CECs) are currently an unregulated class of contaminants with increasing global presence and awareness. However, the management of CECs in water bodies is particularly challenging due to the difficulty in detection and their recalcitrant degradation by conventional means. Light-based oxidation processes are viable options for such application. Light-driven oxidation processes use light as an irradiation source to generate oxidative species for the degradation of emerging contaminants. The key few technologies available are discussed in the review article: photo/Fenton, photocatalysis, photolysis, UV/Ozone. Herein, a cost–benefit analysis on various light-based processes was conducted to access the suitability for CECs degradation. It was found that the UV/Ozone process might not be suitable due to the complication with pH adjustments and limited light wavelength. It was found that EEO values were in this sequence: UV only > UV/combination > photocatalyst > UV/O3 > UV/Fenton > solar/Fenton. The solar/Fenton process has the least computed EEO < 5 kWh m−3 and great potential for further development. Newer innovations such as solar/catalyst can also be explored with potentially lower EEO values. Light-based processes could also be used to detect CECs in surface water. Hence forming the 2 pronged approach for CEC management.
1. Introduction
2. Overview of Light-Driven Processes
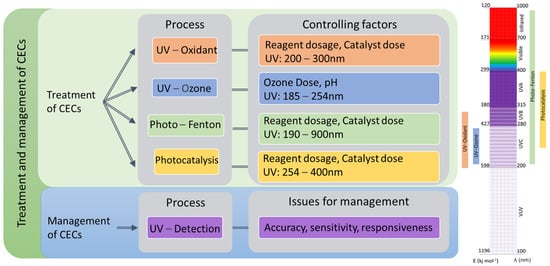
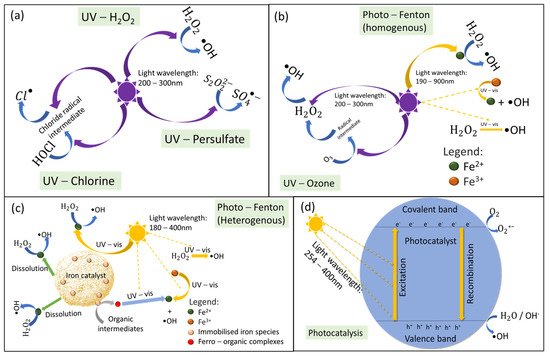
2.1. Mechanism of Light-Driven Processes
3. Cost–Benefit Analysis of Light-Driven AOPs on the Treatment of CECs
4. Light-based detection methods of CECs
A cheaper alternative for the detection of CECs is through the use of light-based spectroscopy. Light-driven detection techniques have been widely proven to be useful in characterizing natural organic matter (NOM) in natural water, drinking water and wastewater. Adsorption of light measures the ‘missing’ wavelength of light that is shone on the water sample. Light-driven detection is based on the absorption of light by organic compounds which results in the excitation of the electrons from the ground state to a higher energy state. The energy difference of each ground state and excitation state pair corresponds to an absorption band. Compounds that contain aromatic rings and double bonds can absorb energy in the form of ultraviolet light to excite the electrons to higher anti-bonding molecular orbitals. UV absorbance, especially at 254 nm is one of the most widely used surrogate parameters to quantify NOM reactivity. Previous studies also found that ●OH radicals tend to react with large molecules with various reaction sites, aromatic compounds, electron-rich organic moieties. Hence, UV254 nm or UV280 nm could be used as indicators to predict the removal of CECs from UV/H2O2. Newer forms of chemiluminescence use enzyme-linked immunosorbent assays (ELISA) and time-resolved fluoroimmunoassays to detect CECs with a high degree of accuracy. Whereas for the excitation and emission type, a known light source is shone on the sample and the measured signals would be the excitation wavelength vs. emission wavelength vs. fluorescence intensity. The EEM could be used to discriminate different groups of NOM based on the difference in light emission and excitation of fluorophores. NOMs with certain molecular structures are reported to have fluorescent properties in a wide range of excitation/ emission wavelengths. Lastly, the physicochemical signal detection relies on the physio-chemical reaction to light onto the sample and is highly dependent on the compounds being monitored. Infrared spectroscopy was also explored to detect a low concentration of CECs. Recently, various core-shell nanostructures have been widely developed to enhance the surface-enhanced Raman scattering (SERS) techniques for the detection of pesticides. Newer methodology like Surface plasmon resonance (SPR) was also demonstrated to have the potential for CEC detection.
Detailed examples of the newer innovation for ligh-based CECs could be found in the article listed.
References
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156.
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2017, 90, 398–428.
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966.
- Choi, Y.Y.; Baek, S.R.; Kim, J.L.; Choi, J.W.; Hur, J.; Lee, T.U.; Park, C.J.; Lee, B.J. Characteristics and Biodegradability of Wastewater Organic Matter in Municipal Wastewater Treatment Plants Collecting Domestic Wastewater and Industrial Discharge. Water (Switz.) 2017, 9, 409.
- Ahearn, A. A Regrettable Substitute: The Story of GenX. Pod. Res. Perspect. 2019, 2019.
- Sharma, S.; Tolley, H.D.; Farnsworth, P.B.; Lee, M.L. LED-based UV absorption detector with low detection limits for capillary liquid chromatography. Anal. Chem. 2015, 87, 1381–1386.
- Safari, G.H.; Yetilmezsoy, K.; Mahvi, A.H.; Zarrabi, M. Post-treatment of secondary wastewater treatment plant effluent using a two-stage fluidized bed bioreactor system. J. Environ. Health Sci. Eng. 2013, 11, 10.
- Calvert, J.G.; Pms, J.N., Jr. Photochemistry; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1967; Volume 6, p. 601.
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637.
- Yu, H.W.; Park, M.; Wu, S.; Lopez, I.J.; Ji, W.; Scheideler, J.; Synder, S.A. Strategies for selecting indicator compounds to assess attenuation of emerging contaminants during UV advanced oxidation processes. Water Res. 2019, 166, 115030.
- Jankunaite, D.; Tichonovas, M. Removal of Diclofenac, Ketoprofen, and Carbamazepine from Simulated Drinking Water by Advanced Oxidation in a Model Reactor. Water Air Soil Pollut. 2017, 228, 353.
- Guo, K.; Wu, Z.; Yan, S.; Yao, B.; Song, W.; Hua, Z.; Zhang, X.; Kong, X.; Li, X.; Fang, J. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water Res. 2018, 147, 184–194.
- Wardenier, N.; Liu, Z.; Nikiforov, A.; van Hulle, S.W.H.; Leys, C. Micropollutant elimination by O3, UV and plasma-based AOPs: An evaluation of treatment and energy costs. Chemosphere 2019, 234, 715–724.
- Davididou, K.; McRitchie, C.; Antonopoulou, M.; Konstantinou, I.; Chatzisymeon, E. Photocatalytic degradation of saccharin under UV-LED and blacklight irradiation. J. Chem. Technol. Biotechnol. 2018, 93, 269–276.
- Sgroi, M.; Snyder, S.A.; Roccaro, P. Comparison of AOPs at pilot scale: Energy costs for micro-pollutants oxidation, disinfection by-products formation and pathogens inactivation. Chemosphere 2020, 273, 128527.
- Expósito, A.J.; Patterson, D.A.; Monteagudo, J.M.; Durán, A. Sono-photo-degradation of carbamazepine in a thin falling film reactor: Operation costs in pilot plant. Ultrason. Sonochem. 2017, 34, 496–503.





