Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Magnus Ingelman-Sundberg | + 2250 word(s) | 2250 | 2021-08-10 10:54:42 | | | |
| 2 | Lily Guo | Meta information modification | 2250 | 2021-09-02 02:53:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ingelman-Sundberg, M. CYP2E1. Encyclopedia. Available online: https://encyclopedia.pub/entry/13795 (accessed on 07 February 2026).
Ingelman-Sundberg M. CYP2E1. Encyclopedia. Available at: https://encyclopedia.pub/entry/13795. Accessed February 07, 2026.
Ingelman-Sundberg, Magnus. "CYP2E1" Encyclopedia, https://encyclopedia.pub/entry/13795 (accessed February 07, 2026).
Ingelman-Sundberg, M. (2021, September 01). CYP2E1. In Encyclopedia. https://encyclopedia.pub/entry/13795
Ingelman-Sundberg, Magnus. "CYP2E1." Encyclopedia. Web. 01 September, 2021.
Copy Citation
CYP2E1 is one of the fifty-seven cytochrome P450 genes in the human genome and is highly conserved. CYP2E1 is a unique P450 enzyme because its heme iron is constitutively in the high spin state, allowing direct reduction of, e.g., dioxygen, causing the formation of a variety of reactive oxygen species and reduction of xenobiotics to toxic products.
NASH
NAFLD
liver
1. Introduction
Early studies of the metabolism of ethanol, independent of alcohol dehydrogenase (ADH), were performed using a mutant strain of deermouse (Peromyscus maniculatus) that is genetically deficient in low-Km ADH. Nonetheless, these mice were found to eliminate ethanol by a previously unidentified enzyme system. One of the enzyme candidates proposed was a cytochrome P450 active in ethanol oxidation in liver microsomes [1], which was also metabolized other short chain alcohols [2] and appeared to be inducible by ethanol treatment in animal models [3].
The exact mechanisms of P450-dependent ethanol oxidation were initially unclear but were associated with the production of reactive oxidative species (ROS), including hydroxyl radicals, which can indirectly oxidize ethanol [4][5]. The finding of an ethanol-dependent increase in P450-mediated oxidation [3][6] was important for the isolation of a specific form of ethanol inducible P450 and to understand the direct P450-mediated oxidation of ethanol. One such enzyme was purified from ethanol- and benzene-induced rabbits and from ethanol-treated rats [7][8]. This enzyme had the highest ethanol oxidation capacity of several different P450 forms isolated [7]. The corresponding cDNA was cloned in 1987 [9] and the enzyme was named cytochrome P450 2E1 (CYP2E1) in 1991 [10].
2. Expression, Functions and Cellular Fate of CYP2E1
CYP2E1 is well conserved across mammalian species, indicating an important physiological function. In humans, the CYP2E1 gene has nine exons located on chromosome 10 and spans 11,413 base pairs [11]. Substantial inter-ethnic polymorphisms exist in CYP2E1. However, only very rare variants causing amino acid shifts have been described [12], and despite several epidemiological studies there is no clear evidence that any polymorphic variants have any functional relevance [13][14]. One recent study suggested a link between CYP2E1-333A > T and NASH, the authors showed increased inflammation and NASH in patient biopsies with the TA allele, largely mediated by a small increase in interferon-inducible protein 10 [15]. However, because only a relatively low number of patients were studied, much credence cannot be given to these findings unless they are reproduced independently. In liver, CYP2E1 is expressed mainly in the endoplasmic reticulum (ER) of the hepatocytes, but is also found in the hepatic Kupffer cells [16][17][18]. Hepatic CYP2E1 is also present in the mitochondria, by translocation, following expression in the nuclear genome [19][20][21] and the plasma membrane [19][22][23][24]. However, the magnitude of induction of CYP2E1 in mitochondria is smaller than that of ER-resident CYP2E1.
CYP2E1 metabolizes a variety of small, hydrophobic substrates and drugs (reviewed in [13][14][21][25]). It is responsible for the metabolism of many toxic or carcinogenic chemicals, including chloroform and benzene, and drugs, such as paracetamol, salicylic acid and several inhalational anesthetics (e.g., isoflurane, sevoflurane and halothane). It therefore follows that conditions which elevate the expression of CYP2E1 can increase the damage caused by conversion of drugs to toxic intermediates [26]. The bioactivation of several pre-carcinogens by CYP2E1 has been discussed in relation to the development of cancers, particularly hepatocellular carcinoma (HCC) [27][28]. In addition, CYP2E1 metabolizes endogenous substances including acetone, acetol, steroids and polyunsaturated fatty acids, such as linoleic acid and arachidonic acid to generate ω-hydroxylated fatty acids [29][30][31][32]. CYP2E1 also metabolizes ethanol and other short chain alcohols, however its contribution to the overall ethanol clearance is low and P450-dependent alcohol metabolism does not influence overall ethanol clearance in rats [33]. Despite its importance as a metabolic enzyme the crystal structure and binding sites were only determined relatively recently, in 2008 [34][35].
CYP2E1 is regulated by multiple, distinct mechanisms at the transcriptional, post-transcriptional, translational, and post-translational levels (Figure 1) [36][37][38][39][40]. CYP2E1 expression is elevated in response to a variety of physiological and pathophysiological conditions, such as starvation and uncontrolled diabetes, and also by ethanol, acetone and several other low molecular weight substrates [7][29][41][42][43][44][45]. However, it is primarily regulated at the post-transcriptional and post-translational levels [46]. Substrate-induced enzyme stabilization is the most important regulatory mechanism for CYP2E1 [47][48]; substrates and other chemicals binding to the substrate binding region stabilize the enzyme and prevent degradation by the proteasome-ER complex [38][48][49], involving the UBC7/gp78 and UbcH5a/CHIP E2-E3 ubiquitin ligases [50]. Thus, ER-mediated degradation is mainly active on CYP2E1 in the absence of substrates, whereas ligand-stabilized CYP2E1 is degraded more slowly by the autophagic-lysosomal pathway [51]. Due to its important physiological functions, the expression of CYP2E1 is under tight homeostatic control and multiple endogenous factors regulate CYP2E1 mRNA stability and protein expression including hormones (such as insulin, glucagon, growth hormone, adiponectin and leptin), growth factors (such as epidermal growth factor and hepatocyte growth factor) and various cytokines (reviewed in [40]).
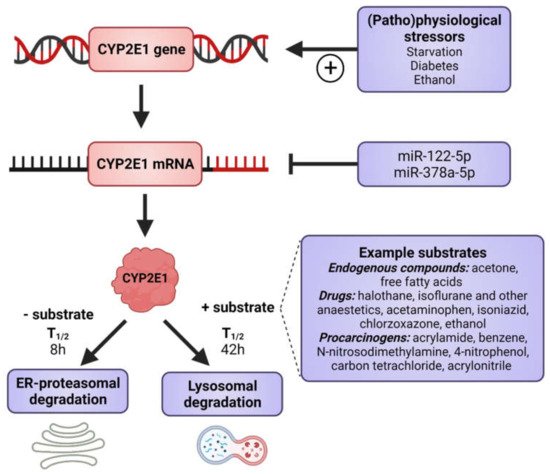
Figure 1. Factors that induce CYP2E1 expressions. CYP2E1 can be induced by multiple mechanisms in transcriptional, post-transcriptional, translational, and post-translational levels. Some physiological and pathophysiological conditions, such as starvation and uncontrolled diabetes, increase CYP2E1 on the transcriptional level. Many hormones and cytokines regulate CYP2E1 on the mRNA and protein expression levels. Enzyme substrates stabilize the enzyme, preventing degradation by the ER proteasome and the enzyme degradation instead takes place via the autophagosome-lysosomal pathway. Figure made using BioRender.
As previously mentioned, the CYP2E1 gene is highly conserved and no functionally important genetic variants have been described. This indicates an important endogenous role, which is supported by our findings, wherein the knockdown of human CYP2E1 expression in the in vivo relevant three-dimensional (3D) liver spheroids [52] causes dramatic cell death in hepatocytes (unpublished observations from our lab). It is clear that CYP2E1 has important effects during catabolic conditions as the gene is transcriptionally induced during starvation [41]. The hepatic production of glucose is essential under starvation conditions with the primary supply of brain glucose, and about 10% of plasma glucose originating from acetone [53]. CYP2E1 readily oxidizes acetone to acetol [36], which is subsequently converted to pyruvate and then to glucose during gluconeogenesis. In addition, during conditions of starvation energy supply from fatty acids is essential and CYP2E1 is efficient in the ω-oxidation of fatty acids.
3. CYP2E1 in ALD
ALD is the most frequent liver disease in Europe, causing approximately 500,000 deaths per year, HCC resulting from pathological changes in the liver contributes highly to this death rate. There are strong genetic determinants in the development of ALD, e.g., PNPLA3, TM6SF2, and only approximately 10–20% of alcoholics develop cirrhosis of the liver [54].
ALD and NAFLD have several common mechanisms; generally, fibrosis occurs in response to enhanced ROS levels, lipid mediators and pro-inflammatory cytokines. Thus, ALD and NAFLD are mechanistically similar and share histopathological features, particularly in terms of CYP2E1 induction and oxidative stress (reviewed in [55]). In addition, they share common genetic risk factors.
CYP2E1 is the most relevant CYP in ALD [56], it is highly inducible, has high catalytic activity for ethanol [25] and is prone to futile cycling in the absence of substrate to produce ROS [57]. CYP2E1 is regarded as a ‘leaky’ enzyme due to loose coupling of the CYP redox cycle, permitted by the constitutively high-spin state of the heme iron and, therefore, it has a great capacity to produce oxyradicals and initiate lipid peroxidation [58][59][60][61][62]. Thus, CYP2E1 may be important in mediating the effects of ethanol on ALD via increased lipid peroxidation [58].
In order to study the link between CYP2E1 and ALD, Morgan et al., generated CYP2E1 transgenic mice which exhibited increased serum ALT levels, higher histological scoring and ballooning hepatocytes with alcohol diet [63]. Using a transgenic mouse model expressing extra copies of human CYP2E1, Butura et al., showed increased liver injury and expression of stress related genes with alcohol diet [64]. Microarray analyses revealed that enhanced expression of structural genes, particularly cytokeratin 8 and 18, may be related to the observed pathology and they were suggested as biomarkers for ALD [64]. JunD, part of the transcription factor complex AP-1, was induced by CYP2E1 and alcohol, and its expression correlated with the degree of liver injury. This transcription factor complex is also linked to increased macrophage activation. Furthermore, JunD also has a role in hepatic stellate cell activation and regulates the cytokine interleukin 6 [64].
A second approach is to study CYP2E1 knockout mice in comparison to wild-type mice. Abdelmegeed et al., showed that that aged wild-type mice had increased hepatocyte vacuolation, ballooning, degeneration, and inflammatory cell infiltration compared with CYP2E1-null mice [65]. They also found that the aged wild-type mice had increased hepatocyte apoptosis, hepatic fibrosis, levels of hepatic hydrogen peroxide, lipid peroxidation, protein carbonylation, nitration and oxidative DNA damage, indicating an endogenous role for CYP2E1 for these events.
Another approach to study the influence of CYP2E1 on ALD involves the use of CYP2E1 inhibitors. Chlormethiazole is a specific CYP2E1 inhibitor [66] and has a pronounced inhibitory effect on ALD in the intra-gastric alcohol rat model [67]. Similar experiments were also conducted using diallyl sulfide and phenylethyl isothiocyanate as CYP2E1 inhibitors demonstrating a protective effect against hydroxyradical formation from ethanol and lipid peroxidation and inhibition of some pathological scores [68][69].
Together, these studies indicate a significant contribution of alcohol-dependent induction of CYP2E1 for the development of ALD [70]. The major cause is its ability to increase ROS formation after ethanol treatment, but other factors controlling the redox properties of the liver also contribute to the observed pathology.
Intestinal CYP2E1 in ALD
In addition to causing gut dysbiosis, alcohol increases CYP2E1 levels and nitroxidative stress in the intestinal epithelium similarly as in the liver [71][72][73][74][75]. This causes intestinal leakiness followed by increased circulating endotoxin levels [73][76][77][78]. Endotoxin can initiate a hepatic necroinflammatory cascade starting from increased levels of NF-κB and release of inflammatory cytokines, such as TNF-α by Kupffer cells [76][79][80]. These results suggest a role of intestinal protein nitration in mediating alcohol-induced gut leakiness and subsequent hepatic injury in a CYP2E1-dependent manner. Even though fatty acids induce CYP2E1 in liver, as discussed later in this review, it is unclear whether this holds for intestinal CYP2E1 and increases gut leakiness playing a role in the development of NAFLD. NASH patients exhibit increased gut leakiness [81] and dysbiosis (reviewed in [82]) and gut inflammation and dysbiosis augments hepatic inflammation and fibrogenesis in mouse NASH models. However, the role of intestinal CYP2E1 and oxidative stress for gut leakiness was not tested [81][83][84]. It has been hypothesized that ethanol produced by microbiota fermenting dietary sugars could cause dysbiosis, increased CYP2E1 levels and nitroxidative stress in NAFLD patients (reviewed in [82]) and maybe endotoxin levels are thus lower in patients with alcoholic cirrhosis compared with non-alcoholic cirrhosis [78].
4. CYP2E1 in NAFLD
NAFLD has been known for over 40 years; despite this, the underlying mechanisms remain poorly understood [85]. NAFLD refers to a continuum of liver diseases beginning with non-alcoholic fatty liver (NAFL), an accumulation of hepatic lipids not explained by alcohol consumption. In some cases, this can progress towards non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis and eventually HCC. Most NAFLD patients are obese and exhibit mild systemic inflammation, which induces insulin resistance and plays a role in the mechanism of liver damage [86][87][88][89]. NASH encompasses varying degrees of liver injury and is now recognized as the hepatic component of metabolic syndrome [90][91][92][93].
Liver cirrhosis is part of alcoholic steatohepatitis (ASH) and NASH, for which the only curative solution is often liver transplantation, and can progress to HCC in 3–4% of cases. The major causes of liver fibrosis are: hepatitis C infection (33%), alcohol (30%) and NASH (23%) [94]. The individual risks for liver cirrhosis caused by NASH and alcohol are largely determined by genetic risk factors, with polymorphisms in PNPLA3 and TM6SF2 and also MBOAT7, HSD17B13 and PCKS7 being the major common genetic determinants [95].
The multiple parallel hit model aims to explain the initiation and progression of NAFLD and has, in recent years, superseded the more simplistic two-hit model [92][96]. It suggests that multiple concurrent environmental and genetic insults, such as insulin resistance, oxidative stress-induced mitochondrial dysfunction, ER stress, endotoxin-induced TLR4-dependent release of inflammatory cytokines, and free fatty acid (FFA) accumulation combine to produce cell death and damage [97]. Oxidative stress mediated by ROS likely plays a primary role as the initiator of hepatic and extrahepatic damage and can cause damage in myriad ways by peroxidation of cellular macromolecules [98]. Oxidative stress can lead to lipid accumulation both directly and indirectly, most simplistically, ROS can peroxidate cellular lipids. Presence of these peroxidated lipids increases post-translational degradation of ApoB, preventing hepatocellular lipid export and leading to lipid accumulation. Alternatively, ROS can directly peroxidate proteins such as ApoB, directly preventing their function and producing a similar effect [99].
The connection between CYP2E1 and NASH was first suggested by Weltman et al., in 1996, where elevated levels of CYP2E1 were observed in steatosis and NASH patients, particularly in the centrilobular region [100] corresponding to the site of maximal hepatocellular injury in NASH [41][101]. This connection is based on the high propensity of CYP2E1 to generate ROS, even in the absence of substrates (Figure 2). In addition, CYP2E1 levels are elevated in obesity, steatosis and NASH in both humans and rodents [102][103][104].
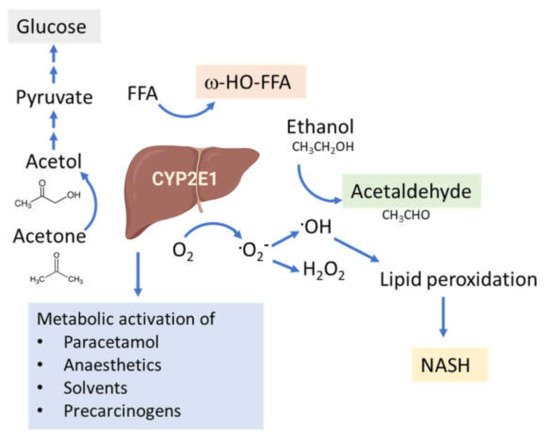
Figure 2. Functions of the CYP2E1 enzyme. CYP2E1 has a role in normal physiological homeostasis by metabolizing endogenous and exogenous compounds. Major endogenous functions are ω-hydroxylation of FFA and oxidation of acetone to precursors of gluconeogenesis. Increased amounts of CYP2E1 results in increased lipid peroxidation and ROS production and is associated with the progression of NAFLD to NASH.
References
- Shigeta, Y.; Nomura, F.; Iida, S.; Leo, M.A.; Felder, M.R.; Lieber, C.S. Ethanol metabolism in vivo by the microsomal ethanol-oxidizing system in deermice lacking alcohol dehydrogenase (ADH). Biochem. Pharmacol. 1984, 33, 807–814.
- Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal alcohol-oxidizing system. Affinity for methanol, ethanol, propanol, and butanol. J. Biol. Chem. 1975, 250, 7397–7404.
- Morgan, E.T.; Devine, M.; Skett, P. Changes in the rat hepatic mixed function oxidase system associated with chronic ethanol vapor inhalation. Biochem. Pharmacol. 1981, 30, 595–600.
- Ingelman-Sundberg, M.; Johansson, I. The mechanism of cytochrome P-450-dependent oxidation of ethanol in reconstituted membrane vesicles. J. Biol. Chem. 1981, 256, 6321–6326.
- I Cederbaum, A.; Dicker, E.; Gutteridge, J.M.; Smith, A.; A Clejan, L.; Groebler, L.K.; Liu, J.; Shanu, A.; Codd, R.; Witting, P.K.; et al. Inhibition of microsomal oxidation of alcohols and of hydroxyl-radical-scavenging agents by the iron-chelating agent desferrioxamine. Biochem. J. 1983, 210, 107–113.
- Teschke, R.; Moreno, F.; Petrides, A.S. Hepatic microsomal ethanol oxidizing system (MEOS): Respective roles of ethanol and carbohydrates for the enhanced activity after chronic alcohol consumption. Biochem. Pharmacol. 1981, 30, 1745–1751.
- Koop, D.R.; Morgan, E.; E Tarr, G.; Coon, M.J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J. Biol. Chem. 1982, 257, 8472–8480.
- Ingelman-Sundberg, M.; Hagbjork, A.L. On the significance of the cytochrome P-450-dependent hydroxyl radical-mediated oxygenation mechanism. Xenobiotica 1982, 12, 673–686.
- Khani, S.C.; Zaphiropoulos, P.; Fujita, V.S.; Porter, T.D.; Koop, D.R.; Coon, M.J. cDNA and derived amino acid sequence of ethanol-inducible rabbit liver cytochrome P-450 isozyme 3a (P-450ALC). Proc. Natl. Acad. Sci. USA 1987, 84, 638–642.
- Nebert, D.W.; Nelson, D.R.; Coon, M.J.; Estabrook, R.W.; Feyereisen, R.; Fujii-Kuriyama, Y.; Gonzalez, F.J.; Guengerich, F.P.; Gunsalus, I.C.; Johnson, E.F.; et al. The P450 Superfamily: Update on New Sequences, Gene Mapping, and Recommended Nomenclature. DNA Cell Biol. 1991, 10, 1–14.
- Umeno, M.; McBride, O.W.; Yang, C.S.; Gelboin, H.V.; Gonzalez, F.J. Human ethanol-inducible P450IIE1: Complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expression. Biochemistry 1988, 27, 9006–9013.
- Gaedigk, A.; Sangkuhl, K.; Whirl-Carrillo, M.; Twist, G.P.; Klein, T.E.; Miller, N.A. The PharmVar Steering Committee The Evolution of PharmVar. Clin. Pharmacol. Ther. 2019, 105, 29–32.
- Tanaka, E.; Terada, M.; Misawa, S. Cytochrome P450 2E1: Its clinical and toxicological role. J. Clin. Pharm. Ther. 2000, 25, 165–175.
- Ronis, M.J.J.; Lindros, K.O.; Ingelman-Sundberg, M. The CYP2E subfamily. Cytochromes P 1996, 450, 211–239.
- Ma, H.; Chen, S.; I Zheng, K.; Yu, Y.; Wang, X.; Tang, L.; Li, G.; Rios, R.S.; Huang, O.; Zheng, X.; et al. TA allele of rs2070673 in the CYP2E1 gene is associated with lobular inflammation and nonalcoholic steatohepatitis in patients with biopsy-proven nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021.
- Koop, D.R.; Chernosky, A.; Brass, E.P. Identification and induction of cytochrome P450 2E1 in rat Kupffer cells. J. Pharmacol. Exp. Ther. 1991, 258, 1072–1076.
- Koivisto, T.; Mishin, V.M.; Mak, K.M.; Cohen, P.A.; Lieber, C.S. Induction of Cytochrome P-4502E1 by Ethanol in Rat Kupffer Cells. Alcohol. Clin. Exp. Res. 1996, 20, 207–212.
- Ye, Q.; Wang, X.; Wang, Q.; Xia, M.; Zhu, Y.; Lian, F.; Ling, W. Cytochrome P4502E1 inhibitor, chlormethiazole, decreases lipopolysaccharide-induced inflammation in rat Kupffer cells with ethanol treatment. Hepatol. Res. 2013, 43, 1115–1123.
- Neve, E.P.A.; Ingelman-Sundberg, M. Molecular Basis for the Transport of Cytochrome P450 2E1 to the Plasma Membrane. J. Biol. Chem. 2000, 275, 17130–17135.
- Neve, E.P.; Ingelman-Sundberg, M. A soluble NH2-terminally truncated catalytically active form of rat cytochrome P450 2E1 targeted to liver mitochondria. FEBS Lett. 1999, 460, 309–314.
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544.
- Loeper, J.; Descatoire, V.; Maurice, M.; Beaune, P.; Feldmann, G.; Larrey, D.; Pessayre, D. Presence of functional cytochrome P-450 on isolated rat hepatocyte plasma membrane. Hepatology 1990, 11, 850–858.
- Loeper, J.; Descatoire, V.; Maurice, M.; Beaune, P.; Belghiti, J.; Houssin, D.; Ballet, F.; Feldmann, G.; Guengerich, F.; Pessayre, D. Cytochromes P-450 in human hepatocyte plasma membrane: Recognition by several autoantibodies. Gastroenterology 1993, 104, 203–216.
- Neve, E.P.; Eliasson, E.; Pronzato, M.A.; Albano, E.; Marinari, U.; Ingelman-Sundberg, M. Enzyme-Specific Transport of Rat Liver Cytochrome P450 to the Golgi Apparatus. Arch. Biochem. Biophys. 1996, 333, 459–465.
- Koop, D.R. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992, 6, 724–730.
- Massart, J.; Begriche, K.; Fromenty, B. Cytochrome P450 2E1 should not be neglected for acetaminophen-induced liver injury in metabolic diseases with altered insulin levels or glucose homeostasis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101470.
- Seitz, H.K. The role of cytochrome P4502E1 in the pathogenesis of alcoholic liver disease and carcinogenesis. Chem. Interact. 2020, 316, 108918.
- Seitz, H.K.; Mueller, S. Alcohol and Cancer: An Overview with Special Emphasis on the Role of Acetaldehyde and Cytochrome P450 2E1. Adv. Exp. Med. Biol. 2014, 815, 59–70.
- Johansson, I.; Eliasson, E.; Norsten, C.; Ingelman-Sundberg, M. Hydroxylation of acetone by ethanol- and acetone-inducible cytochrome P-450 in liver microsomes and reconstituted membranes. FEBS Lett. 1986, 196, 59–64.
- Song, B.; Cederbaum, A.I. Ethanol-Inducible Cytochrome P450 (CYP2E1): Biochemistry, Molecular Biology and Clinical Relevance: 1996 Update. Alcohol. Clin. Exp. Res. 1996, 20, 138a–146a.
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. Mol. Mech. Mutagen. 2005, 569, 101–110.
- Correia, M.A.; Kwon, D. Why Hepatic CYP2E1-Elevation by Itself Is Insufficient for Inciting NAFLD/NASH: Inferences from Two Genetic Knockout Mouse Models. Biology 2020, 9, 419.
- Badger, T.M.; Ronis, M.J.J.; Ingelman-Sundberg, M.; Hakkak, R. Inhibition of CYP2E1 Activity does not Abolish Pulsatile Urine Alcohol Concentrations During Chronic Alcohol Infusions. JBIC J. Biol. Inorg. Chem. 1995, 230, 914–919.
- Porubsky, P.R.; Meneely, K.M.; Scott, E.E. Structures of Human Cytochrome P-450 2E1. J. Biol. Chem. 2008, 283, 33698–33707.
- Porubsky, P.R.; Battaile, K.; Scott, E.E. Human Cytochrome P450 2E1 Structures with Fatty Acid Analogs Reveal a Previously Unobserved Binding Mode. J. Biol. Chem. 2010, 285, 22282–22290.
- Koop, D.R.; Crump, B.L.; Nordblom, G.D.; Coon, M.J. Immunochemical evidence for induction of the alcohol-oxidizing cytochrome P-450 of rabbit liver microsomes by diverse agents: Ethanol, imidazole, trichloroethylene, acetone, pyrazole, and isoniazid. Proc. Natl. Acad. Sci. USA 1985, 82, 4065–4069.
- Koop, D.R.; Tierney, D.J. Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. BioEssays 1990, 12, 429–435.
- Song, B.J.; Matsunaga, T.; Hardwick, J.P.; Park, S.S.; Veech, R.L.; Yang, C.S.; Gelboin, H.V.; Gonzalez, F.J. Stabilization of Cytochrome P450j Messenger Ribonucleic Acid in the Diabetic Rat. Mol. Endocrinol. 1987, 1, 542–547.
- Yun, Y.P.; Casazza, J.P.; Sohn, D.H.; Veech, R.L.; Song, B.J. Pretranslational activation of cytochrome P450IIE during ketosis induced by a high fat diet. Mol. Pharmacol. 1992, 41, 474–479.
- Novak, R.F.; Woodcroft, K.J. The alcohol-inducible form of cytochrome P450 (CYP2E1): Role in toxicology and regulation of expression. Arch. Pharml. Res. 2000, 23, 267–282.
- Johansson, I.; Lindros, K.O.; Eriksson, H.; Ingelman-Sundberg, M. Transcriptional control of CYP2E1 in the perivenous liver region and during starvation. Biochem. Biophys. Res. Commun. 1990, 173, 331–338.
- Yap, C.G.; Zaini, A.; Othman, I. Targeted CYP2E1 quantification and its correlation to currently acceptable clinical biochemical indices. J. Biol. Res. 2016, 23, 15.
- Lee, S.S.; Buters, J.; Pineau, T.; Fernandez-Salguero, P.M.; Gonzalez, F.J. Role of CYP2E1 in the Hepatotoxicity of Acetaminophen. J. Biol. Chem. 1996, 271, 12063–12067.
- Johansson, I.; Ekstroem, G.; Scholte, B.; Puzycki, D.; Joernvall, H.; Ingelman-Sundberg, M. Ethanol-, fasting-, and aceton-inducible cytochromes P-450 in rat liver: Regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry 1988, 27, 1925–1934.
- Koop, D.R.; Coon, M.J. Purification of liver microsomal cytochrome P-450 isozymes 3a and 6 from imidazole-treated rabbits. Evidence for the identity of isozyme 3a with the form obtained by ethanol treatment. Mol. Pharmacol. 1984, 25, 494–501.
- Song, B.J.; Gelboin, H.V.; Park, S.S.; Yang, C.S.; Gonzalez, F.J. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J. Biol. Chem. 1986, 261, 16689–16697.
- Eliasson, E.; Johansson, I.; Ingelman-Sundberg, M. Substrate-, hormone-, and cAMP-regulated cytochrome P450 degradation. Proc. Natl. Acad. Sci. USA 1990, 87, 3225–3229.
- Eliasson, E.; Mkrtchian, S.; Ingelman-Sundberg, M. Hormone- and substrate-regulated intracellular degradation of cytochrome P450 (2E1) involving MgATP-activated rapid proteolysis in the endoplasmic reticulum membranes. J. Biol. Chem. 1992, 267, 15765–15769.
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738.
- Wang, Y.; Guan, S.; Acharya, P.; Koop, D.R.; Liu, Y.; Liao, M.; Burlingame, A.L.; Correia, M.A. Ubiquitin-dependent Proteasomal Degradation of Human Liver Cytochrome P450 2E1. J. Biol. Chem. 2011, 286, 9443–9456.
- Ronis, M.J.J.; Johansson, I.; Hultenby, K.; Lagercrantz, J.; Glaumann, H.; Ingelman-Sundberg, M. Acetone-regulated synthesis and degradation of cytochrome P4502E2 and cytochrome P4502B1 in rat liver. JBIC J. Biol. Inorg. Chem. 1991, 198, 383–389.
- Bell, C.C.; Hendriks, D.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Puigvert, L.F.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187.
- Owen, O.E.; Patel, M.S.; Block, B.S.B.; Kreulen, T.H.; Reichle, F.A.; Mozzoli, M.A. Gluconeogenesis in normal, cirrhotic, and diabetic humans. In Gluconeogenesis: Its Regulation in Mammalian Species; Wiley: New York, NY, USA, 1976; pp. 533–558.
- Mann, R.E.; Smart, R.G.; Govoni, R. The epidemiology of alcoholic liver disease. Alcohol Res. Health 2003, 27, 209–219.
- Sakaguchi, S.; Takahashi, S.; Sasaki, T.; Kumagai, T.; Nagata, K. Progression of Alcoholic and Non-alcoholic Steatohepatitis: Common Metabolic Aspects of Innate Immune System and Oxidative Stress. Drug Metab. Pharmacokinet. 2011, 26, 30–46.
- Leung, T.-M.; Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 395–398.
- Robertson, G.; Leclercq, I.; Farrell, G.C., II. Cytochrome P-450 enzymes and oxidative stress. Am. J. Physiol. Liver Physiol. 2001, 281, G1135–G1139.
- Ekström, G.; Ingelman-Sundberg, M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem. Pharmacol. 1989, 38, 1313–1319.
- Ingelman-Sundberg, M.; Johansson, I.; Yin, H.; Terelius, Y.; Eliasson, E.; Clot, P.; Albano, E. Ethanol-inducible cytochrome P4502E1: Genetic polymorphism, regulation, and possible role in the etiology of alcohol-induced liver disease. Alcohol 1993, 10, 447–452.
- Zhukov, A.; Ingelman-Sundberg, M. Relationship between cytochrome P450 catalytic cycling and stability: Fast degradation of ethanol-inducible cytochrome P450 2E1 (CYP2E1) in hepatoma cells is abolished by inactivation of its electron donor NADPH–cytochrome P450 reductase. Biochem. J. 1999, 340, 453–458.
- Bell, L.C.; Guengerich, F.P. Oxidation Kinetics of Ethanol by Human Cytochrome P450 2E1. J. Biol. Chem. 1997, 272, 29643–29651.
- Gorsky, L.D.; Koop, D.R.; Coon, M.J. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J. Biol. Chem. 1984, 259, 6812–6817.
- Morgan, K.; French, S.W.; Morgan, T.R. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology 2002, 36, 122–134.
- Butura, A.; Nilsson, K.; Morgan, K.; Morgan, T.R.; French, S.W.; Johansson, I.; Schuppe-Koistinen, I.; Ingelman-Sundberg, M. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 2009, 50, 572–583.
- Abdelmegeed, M.A.; Choi, Y.; Ha, S.-K.; Song, B.-J. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic. Biol. Med. 2016, 91, 188–202.
- Hu, Y.; Mishin, V.; Johansson, I.; Von Bahr, C.; Cross, A.; Ronis, M.J.; Badger, T.M.; Ingelman-Sundberg, M. Chlormethiazole as an efficient inhibitor of cytochrome P450 2E1 expression in rat liver. J. Pharmacol. Exp. Ther. 1994, 269, 1286–1291.
- Gouillon, Z.-Q.; Lucas, D.; Li, J.; Hagbjork, A.L.; French, B.A.; Fu, P.; Fang, C.; Ingelman-Sundberg, M.; Donohue, T.M.; French, S.W. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc. Soc. Exp. Boil. Med. 2000, 224, 302–308.
- Albano, E.; Clot, P.; Morimoto, M.; Tomasi, A.; Ingelman-Sundberg, M.; French, S.W. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology 1996, 23, 155–163.
- Morimoto, M.; Hagbjork, A.-L.; Wan, Y.-J.Y.; Fu, P.C.; Clot, P.; Albano, E.; Ingelman-Sundberg, M.; French, S.W. Modulation of experimental alcohol-induced liver disease by cytochrome P450 2E1 inhibitors. Hepatology 1995, 21, 1610–1617.
- Seitz, H.; Neuman, M. The History of Alcoholic Liver Disease: From an Unrecognized Disease to One of the Most Frequent Diseases in Hepatology. J. Clin. Med. 2021, 10, 858.
- Ballway, J.; Song, B.-J. Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease. Antioxidants 2021, 10, 384.
- Abdelmegeed, M.A.; Banerjee, A.; Jang, S.; Yoo, S.-H.; Yun, J.-W.; Gonzalez, F.J.; Keshavarzian, A.; Song, B.-J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic. Biol. Med. 2013, 65, 1238–1245.
- Abdelmegeed, M.A.; Ha, S.-K.; Choi, Y.; Akbar, M.; Song, B.-J. Role of CYP2E1 in Mitochondrial Dysfunction and Hepatic Injury by Alcohol and Non-Alcoholic Substances. Curr. Mol. Pharmacol. 2017, 10, 207–225.
- Tang, Y.; Forsyth, C.B.; Farhadi, A.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z.; Keshavarzian, A. Nitric Oxide-Mediated Intestinal Injury Is Required for Alcohol-Induced Gut Leakiness and Liver Damage. Alcohol. Clin. Exp. Res. 2009, 33, 1220–1230.
- Forsyth, C.B.; Voigt, R.M.; Shaikh, M.; Tang, Y.; Cederbaum, A.I.; Turek, F.W.; Keshavarzian, A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am. J. Physiol. Liver Physiol. 2013, 305, G185–G195.
- Keshavarzian, A.; Fields, J. Alcoholic liver disease: Is it an “extraintestinal” complication of alcohol-induced intestinal injury? J. Lab. Clin. Med. 2003, 142, 285–287.
- Tamai, H.; Kato, S.; Horie, Y.; Ohki, E.; Yokoyama, H.; Ishii, H. Effect of Acute Ethanol Administration on the Intestinal Absorption of Endotoxin in Rats. Alcohol. Clin. Exp. Res. 2000, 24, 390–394.
- Bode, C.; Kugler, V.; Bode, J. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14.
- Mathurin, P.; Deng, Q.; Keshavarzian, A.; Choudhary, S.; Holmes, E.; Tsukamoto, H. Exacerbation of Alcoholic Liver Injury by Enteral Endotoxin in Rats. Hepatology 2000, 32, 1008–1017.
- Enomoto, N.; Ikejima, K.; Bradford, B.; Rivera, C.; Kono, H.; Brenner, D.A.; Thurman, R.G. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology 1998, 115, 443–451.
- Luther, J.; Garber, J.J.; Khalili, H.; Dave, M.; Bale, S.S.; Jindal, R.; Motola, D.L.; Luther, S.; Bohr, S.; Jeoung, S.W.; et al. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 222–232.e2.
- Jennison, E.; Byrne, C.D. The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 22–43.
- Gäbele, E.; Dostert, K.; Hofmann, C.; Wiest, R.; Schölmerich, J.; Hellerbrand, C.; Obermeier, F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 2011, 55, 1391–1399.
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.; Eisenbarth, S.; Jurczak, M.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nat. Cell Biol. 2012, 482, 179–185.
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Ott, B.J. Nonalcoholic steatohepatitis. Mayo Clinic experiences with a hitherto un-named disease. Mayo Clin. Proc. 1980, 55, 434–438.
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642.
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689.
- Vega, G.L.; Chandalia, M.; Szczepaniak, L.S.; Grundy, S.M. Metabolic correlates of nonalcoholic fatty liver in women and men. Hepatology 2007, 46, 716–722.
- Sanyal, A.J.; Campbell–Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192.
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016.
- Almeda-Valdes, P.; Aguilar-Olivos, N.; Uribe, M.; Méndez-Sánchez, N. Common features of the metabolic syndrome and nonalcoholic fatty liver disease. Rev. Recent Clin. Trials 2015, 9, 148–158.
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048.
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1.
- El-Serag, H.B.; Kanwal, F.; Feng, Z.; Marrero, J.A.; Khaderi, S.; Singal, A.G. Risk Factors for Cirrhosis in Contemporary Hepatology Practices—Findings From the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 2020, 159, 376–377.
- Bianco, C.; Casirati, E.; Malvestiti, F.; Valenti, L. Genetic predisposition similarities between NASH and ASH: Identification of new therapeutic targets. JHEP Rep. 2021, 3, 100284.
- Tilg, H.; Moschen, A. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846.
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128.
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 1–14.
- Pan, M.; Cederbaum, A.I.; Zhang, Y.-L.; Ginsberg, H.N.; Williams, K.J.; Fisher, E.A. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Investig. 2004, 113, 1277–1287.
- Weltman, M.D.; Farrell, G.C.; Liddle, C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 1996, 111, 1645–1653.
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging The Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474.
- Weltman, M.D.; Farrell, G.C.; Hall, P.; Ingelman-Sundberg, M.; Liddle, C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998, 27, 128–133.
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637.
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quiñones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendes, A.; et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004, 106, 261–268.
More
Information
Subjects:
Nutrition & Dietetics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Revisions:
2 times
(View History)
Update Date:
02 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




