
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandrine Etienne-Manneville | + 2547 word(s) | 2547 | 2021-08-04 08:01:22 | | | |
| 2 | Ron Wang | + 344 word(s) | 2891 | 2021-09-01 04:19:17 | | |
Video Upload Options
Cytoplasmic intermediate filaments (IFs), which together with actin and microtubules form the cytoskeleton, are composed of a large and diverse family of proteins. Efforts to elucidate the molecular mechanisms responsible for IF-associated diseases increasingly point towards a major contribution of IFs to the cell’s ability to adapt, resist and respond to mechanical challenges. From these observations, which echo the impressive resilience of IFs in vitro, we here discuss the role of IFs as master integrators of cell and tissue mechanics.
1. Introduction
Tissue integrity, which is necessary for all metazoan life, relies on the ability of cells to adapt their morphology, their interactions, and their function to the conditions of their environment. The cytoskeleton, including actin microfilaments, microtubules, and intermediate filaments (IFs), form essential intracellular networks which support cell shape, cell adhesions and are indispensable for most cellular functions. While the role of actin in cell morphology, motility, and contractility has been extensively studied and the contribution of microtubules to intracellular trafficking, cell polarity, and adhesion dynamics is now well understood, the role of IFs in cell functions and tissue integrity remains unclear. This is partly because, in contrast to the ubiquitously expressed actin and tubulin, IF protein expression varies between cell types and tissues, and IF protein levels can represent anything from 0.3 to 85% of total protein levels in the cell [1][2]. Despite a high level of shared structural features between cytoplasmic IFs such as a head, rod, and tail domain, the more than 70 known IF genes create highly specialized, cell-type-specific networks of polymeric filaments. IFs are subdivided into five subtypes depending on small structural differences, their modes of assembly, and their expression pattern [3]. GFAP, vimentin, synemin, and nestin form the IF network in glia, neurofilaments in neurons, desmin and syncoilin in muscles, keratins in skin, and vimentin in mesenchymal cells. Consequently, the depletion of single IF genes does not always lead to severe phenotypes. However, in humans, mutations in IF genes give rise to a large diversity of diseases commonly characterized by the altered integrity of specific tissues [4]. The lack of associated molecular motors and well-characterized regulators of the assembly/disassembly dynamics further distinguishes IFs from actin and microtubules. These characteristics are probably responsible for our late understanding of IF functions at the cellular and tissue level. Only the painstaking studies of each type of IF’s structural and mechanical properties and their integration at the network, cellular and tissue levels are slowly unraveling the contribution of IFs to the physiology and pathology of multicellular organisms.
2. Intermediate Filaments as Key Players in Tissue and Cellular Mechanics
At the cellular level, studies have progressively confirmed that IF’s function in tissue integrity relies on their contribution to cell resilience under both mechanical stretching and compression. Indeed, the depletion of keratin or vimentin increases the deformability of stretched cells [5][6] ( Figure 1 B,C). Importantly, the loss of vimentin also decreases the viability of cells submitted to stretching [7] ( Figure 1 C). The role of IFs in cellular resistance to deformation by compressive forces appears to be cell-type specific. Under compression, both the depletion of vimentin in human mesenchymal stem cells [8] and the overexpression of vimentin in amoeboid cancer cells [9] reduce cell deformation. This discrepancy may be due to a different initial level of IF protein expression optimized in a cell-type-specific manner to provide cells with different mechanical properties. Alternatively, the difference may result from a cell-type-specific composition of the cytoskeletal network. For example, the depletion of vimentin in compressed highly contractile cells might have a different effect on their deformability compared to depletion in cells with lower contractility. Taken together, these studies show how IFs contribute to cell resilience by limiting cell deformation under mechanical stress and allowing stretched or compressed cells to recover their initial shape without any damage. However, they also suggest that the mechanical resilience of a given cell type in a specific situation may require an optimal level of expression of a specific IF type.
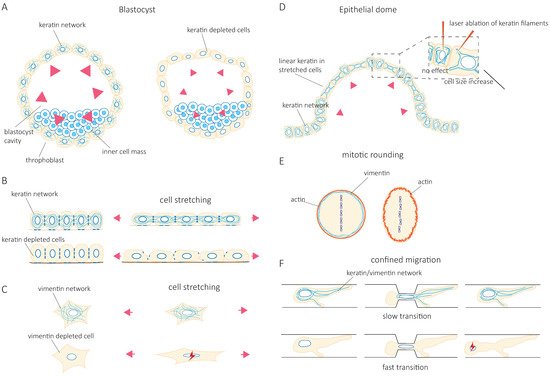
The IF network can also reorganize in response to mechanical stresses [10][11][12][13][14]. Rearrangement of the network is nicely illustrated in epithelial domes. In this three-dimensional in vitro epithelial sheet model, cells undergo extreme deformations while the tension across the epithelial sheet is maintained constant [15]. In fully expanded domes, extremely stretched cells coexist with cells that barely change their shape. The stretched cells are characterized by the formation of unusually straight bundles of keratin IFs, which extend from the nucleus to the plasma membrane. Laser ablation of these IF bundles results in a rapid increase in the cell area [15] ( Figure 1 D). This suggests that keratin IFs in highly stretched cells are load-bearing elements that maintain the reversibility of cell shape after large deformations. At the cellular scale, cell stretching by hypo-osmotic stress partly depolymerizes vimentin and nestin filaments and redistributes them throughout the cells [13]. This rearrangement is essential to cell survival after hypo-osmotic shock, confirming the contribution of IFs to cell mechanical resistance.
Accumulating evidence shows that IF networks are rearranged when cells adapt to external mechanical challenges. Cells can generate contractile or pushing forces to reshape in order to accomplish specific functions such as cell division or migration. To divide, cells must actively generate forces to accommodate their shape changes and overcome mechanical constraints by the surrounding tissue. The IF network reorganizes extensively not only to adapt to but also to promote these changes [16][17][18][19]. As cells round up to facilitate the accurate positioning of the spindle and the correct segregation of chromosomes, cortical tension generated by the actin cortex increases. In HeLa Kyoto cells, vimentin IFs contribute to this increase in cortical tension by relocalizing to the cortex, where they interact with actin to control actin organization [20][21] ( Figure 1 E). In confined environments, the loss of vimentin becomes detrimental to the segregation of chromosomes, and chromosome lagging is often observed [20]. However, besides vimentin IFs, HeLa cells also express keratins, which also reorganize during mitosis and affect the organization of vimentin. Indeed, when cells express nestin, the reorganization of vimentin during cell division is different. In nestin-expressing ovary (CHO) cells, C6-2 glioma, BHK-21 fibroblast, and cerebellar ST15A cells, the vimentin network disassembles at the cleavage furrow and does not localize to the cortex [22][23]. It thus seems that the reorganization of IFs that accompanies the changes in cell mechanics depends on IF proteins that are expressed. The cell-type-specific composition of the IF network needs to be taken into account in future investigations.
Migration in confined environments requires resilient mechanical support to allow cell deformation but prevent damage as they pass through complex environments. IFs appear to provide the essential mechanical support. Keratin knock-out (KO) keratinocytes migrate faster when squeezing through small pores in a Boyden chamber assay. However, they also frequently rupture and die [5] ( Figure 1 F). Similarly, vimentin depletion facilitates the migration of MEFs (mouse embryonic fibroblasts) in confined environments such as microchannels, collagen gels, and small pores [24][25] at the cost of nuclear alterations, nuclear envelop ruptures, and blebs [24] ( Figure 1 F). These observations were also confirmed in studies investigating the amoeboid migration of melanoma cancer cells [9]. Both keratin and vimentin networks appear to provide mechanical support to protect the nucleus against excessive deformations and maintain nuclear homeostasis during confined cell migration [24][25][26]. However, the switch from keratin to vimentin expression observed during the epithelial-to-mesenchymal transition (EMT) suggests that the two IF networks differentially contribute to the cell’s mechanical properties [27][28][29][30]. The microinjection of purified vimentin into MCF-7 epithelial cells changes the cell shape to a mesenchymal cell morphology [28]. Whether this effect is solely due to the mechanical functions of IFs or also reflects their role in intracellular signaling and cell motility remains unclear. While IFs in general, and the organization of the cytoplasmic IF network in particular, are essential to provide cell mechanical resilience, it is tempting to speculate that the mechanical specificity of each type of IF participates in cell-type-specific mechanics. Therefore, the control of IF protein expression may be central to the acquisition of cell-type-specific mechanical behavior adapted to the properties of their microenvironment and the modifications of cell behavior observed in pathological situations.
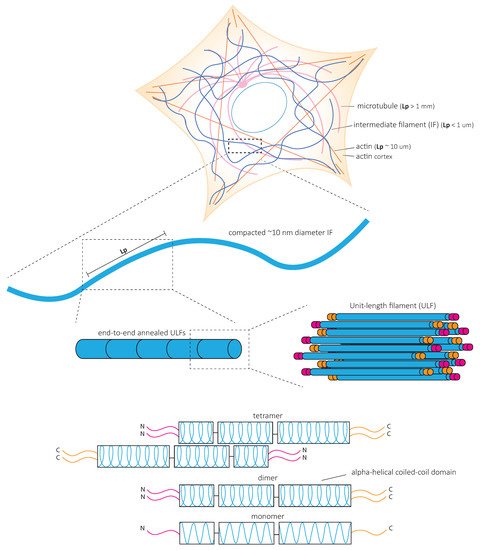
3. Mechanical Properties of IF Networks and Single Filaments In Vitro
In contrast to actin microfilaments and microtubules, which interact with motors such as myosin, dynein, and kinesin, IFs do not bear molecular motors to provide mechanical forces. Instead, it is the fundamental structure of IFs that is at the heart of their mechanical properties ( Figure 2 ). In vitro studies of reconstituted IF networks and single filaments have recently shed light on the structural bases of IF mechanical properties. In this chapter, we recapitulate the results obtained with oscillatory shear rheology experiments on in vitro assembled IF networks which demonstrate their high elastic properties. We discuss how the mechanical properties of IF networks rely on inter-filament interactions ( Figure 3 ) and single filament mechanics ( Figure 4 ) based on biophysical in vitro measurements.
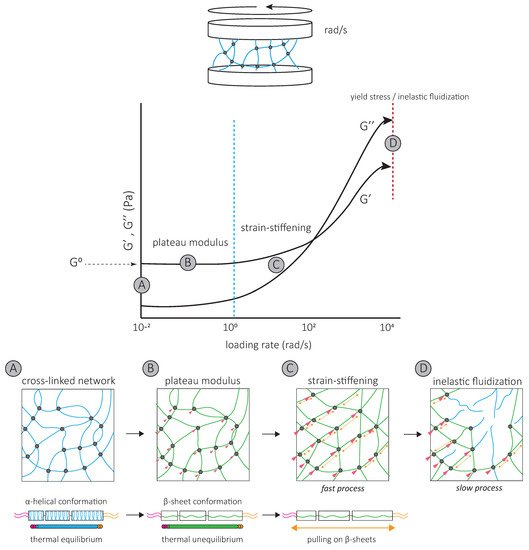
The strain-stiffening behavior of the IF network, observed at high strains, results from further filament stretching between crosslinks ( Figure 3 ) and is completely suppressed by the addition of a non-ionic surfactant [39]. Strain-stiffening is lost in networks formed by tailless filaments [39][40][41][42], showing that the filament C-terminal tails are responsible for the strong, attractive interactions necessary to withstand high stresses [39]. For instance, the characteristic side arms of neurofilament proteins are thought to be involved in the crosslinking of the network and provide resistance at large deformations [43].
The strain-stiffening of the network is limited by the rupture of the network [44][39][40][45] ( Figure 3 ). The stress at which the network ruptures, called the yield stress, is much higher for IF than for actin networks. When the yield stress is reached, a softening of the network is observed, which depends on the nature and degree of inter-filament interactions. The softening of vimentin IF networks is transient, which also distinguishes them from actin networks [46][47], and indicates that filaments are not permanently fractured [48]. Strengthening attractive interactions by the addition of divalent cations [49][40][50][45][51] or permanently crosslinking the network [48] increases the yield stress, suggesting that the softening of IF networks is due to the loss of interactions between IF proteins [48] ( Figure 3 ). The softening of vimentin IF networks is also loading-rate-dependent [48] ( Figure 3 ). At slow loading rates, the disruption of transient C-terminal inter-filament interactions counteracts the strain-stiffening response [48]. Here, the mechanical response is dominated by the disruption of crosslinks which occurs before or at the same time as the stiffening of the network and results in a low yield stress ( Figure 3 ) [48]. At fast loading rates, the strain-stiffening precedes the disruption of crosslinks, and the yield stress is much higher ( Figure 3 ) [48]. Similarly, transient interactions between neurofilament side arms are thought to be responsible for the reorganization of the network following disruption by large prolonged strains [43]. At fast deformations, these interactions provide the IF network with mechanical resilience [43].
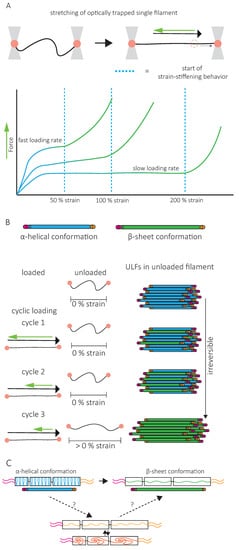
A second specific characteristic of IFs is their high stretchability and strong resistance to breakage. In vitro, single IFs can be stretched about 250% before breakage and can even reach 350% for desmin filaments [52][53]. For low strains up to 100%, a steep increase in force is observed with increasing strain [53][54][55][56]. The force–strain curve plateaus for intermediate strains, and at higher strains, the force further increases, indicating strain-stiffening of the filament [56] ( Figure 4 A). X-ray experiments [57] and mathematical modeling approaches suggest that the steep linear increase in force at low strains results from the elastic stretching of the coiled-coil α-helical domains [56][58][31] ( Figure 4 A). Further stretching of the α-helical domains leads to their transition to β-sheets, reflected by the force plateau at intermediate strains. The stiffening of the filaments at higher strains corresponds to the necessity to exert higher forces to extend β-sheets further ( Figure 4 A). This conformational change and the stretching of single filaments in a β-sheet conformation between filaments in IF networks are essential to the high G 0 and strain-stiffening of the network, respectively ( Figure 3 ).
References
- Herrmann, H.; Häner, M.; Brettel, M.; Müller, S.A.; Goldie, K.N.; Fedtke, B.; Lustig, A.; Franke, W.W.; Aebi, U. Structure and Assembly Properties of the Intermediate Filament Protein Vimentin: The Role of Its Head, Rod and Tail Domains. J. Mol. Biol. 1996, 264, 933–953.
- Colakoğlu, G.; Brown, A. Intermediate Filaments Exchange Subunits along Their Length and Elongate by End-to-End Annealing. J. Cell Biol. 2009, 185, 769–777.
- Vikstrom, K.L.; Borisy, G.G.; Goldman, R.D. Dynamic Aspects of Intermediate Filament Networks in BHK-21 Cells. Proc. Natl. Acad. Sci. USA 1989, 86, 549–553.
- Ngai, J.; Coleman, T.R.; Lazarides, E. Localization of Newly Synthesized Vimentin Subunits Reveals a Novel Mechanism of Intermediate Filament Assembly. Cell 1990, 60, 415–427.
- Coleman, T.R.; Lazarides, E. Continuous Growth of Vimentin Filaments in Mouse Fibroblasts. J. Cell. Sci. 1992, 103 Pt 3, 689–698.
- Vikstrom, K.L.; Lim, S.S.; Goldman, R.D.; Borisy, G.G. Steady State Dynamics of Intermediate Filament Networks. J. Cell Biol. 1992, 118, 121–129.
- Nöding, B.; Herrmann, H.; Köster, S. Direct Observation of Subunit Exchange along Mature Vimentin Intermediate Filaments. Biophys. J. 2014, 107, 2923–2931.
- Aufderhorst-Roberts, A.; Koenderink, G.H. Stiffening and Inelastic Fluidization in Vimentin Intermediate Filament Networks. Soft Matter 2019, 15, 7127–7136.
- Lavenus, S.B.; Tudor, S.M.; Ullo, M.F.; Vosatka, K.W.; Logue, J.S. A Flexible Network of Vimentin Intermediate Filaments Promotes the Migration of Amoeboid Cancer Cells through Confined Environments. J. Biol. Chem. 2020.
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Biol. 2017, 5, 81.
- Laly, A.C.; Sliogeryte, K.; Pundel, O.J.; Ross, R.; Keeling, M.C.; Avisetti, D.; Waseem, A.; Gavara, N.; Connelly, J.T. The Keratin Network of Intermediate Filaments Regulates Keratinocyte Rigidity Sensing and Nuclear Mechanotransduction. Sci. Adv. 2021, 7, eabd6187.
- Pora, A.; Yoon, S.; Dreissen, G.; Hoffmann, B.; Merkel, R.; Windoffer, R.; Leube, R.E. Regulation of Keratin Network Dynamics by the Mechanical Properties of the Environment in Migrating Cells. Sci. Rep. 2020, 10, 4574.
- Li, J.; Gao, W.; Zhang, Y.; Cheng, F.; Eriksson, J.E.; Etienne-Manneville, S.; Jiu, Y. Engagement of Vimentin Intermediate Filaments in Hypotonic Stress. J. Cell Biochem. 2019, 120, 13168–13176.
- van Engeland, N.C.A.; Suarez Rodriguez, F.; Rivero-Müller, A.; Ristori, T.; Duran, C.L.; Stassen, O.M.J.A.; Antfolk, D.; Driessen, R.C.H.; Ruohonen, S.; Ruohonen, S.T.; et al. Vimentin Regulates Notch Signaling Strength and Arterial Remodeling in Response to Hemodynamic Stress. Sci. Rep. 2019, 9, 12415.
- Latorre, E.; Kale, S.; Casares, L.; Gómez-González, M.; Uroz, M.; Valon, L.; Nair, R.V.; Garreta, E.; Montserrat, N.; del Campo, A.; et al. Active Superelasticity in Three-Dimensional Epithelia of Controlled Shape. Nature 2018, 563, 203–208.
- Etienne-Manneville, S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu. Rev. Cell Dev. Biol. 2018, 34.
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the Move: New Developments in Cell Migration. F1000 Res. 2018, 7.
- Lowery, J.; Kuczmarski, E.R.; Herrmann, H.; Goldman, R.D. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J. Biol. Chem. 2015, 290, 17145–17153.
- Nishimura, Y.; Kasahara, K.; Inagaki, M. Intermediate Filaments and IF-Associated Proteins: From Cell Architecture to Cell Proliferation. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 479–493.
- Serres, M.P.; Samwer, M.; Truong Quang, B.A.; Lavoie, G.; Perera, U.; Görlich, D.; Charras, G.; Petronczki, M.; Roux, P.P.; Paluch, E.K. F-Actin Interactome Reveals Vimentin as a Key Regulator of Actin Organization and Cell Mechanics in Mitosis. Dev. Cell 2020, 52, 210–222.e7.
- Duarte, S.; Viedma-Poyatos, Á.; Navarro-Carrasco, E.; Martínez, A.E.; Pajares, M.A.; Pérez-Sala, D. Vimentin Filaments Interact with the Actin Cortex in Mitosis Allowing Normal Cell Division. Nat. Commun. 2019, 10, 4200.
- Chou, Y.-H.; Khuon, S.; Herrmann, H.; Goldman, R.D. Nestin Promotes the Phosphorylation-Dependent Disassembly of Vimentin Intermediate Filaments during Mitosis. Mol. Biol. Cell 2003, 14, 1468–1478.
- Sahlgren, C.M.; Mikhailov, A.; Hellman, J.; Chou, Y.H.; Lendahl, U.; Goldman, R.D.; Eriksson, J.E. Mitotic Reorganization of the Intermediate Filament Protein Nestin Involves Phosphorylation by Cdc2 Kinase. J. Biol. Chem. 2001, 276, 16456–16463.
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin Protects Cells against Nuclear Rupture and DNA Damage during Migration. J. Cell Biol. 2019, 218, 4079–4092.
- Patteson, A.E.; Pogoda, K.; Byfield, F.J.; Mandal, K.; Ostrowska-Podhorodecka, Z.; Charrier, E.E.; Galie, P.A.; Deptuła, P.; Bucki, R.; McCulloch, C.A.; et al. Loss of Vimentin Enhances Cell Motility through Small Confining Spaces. Small 2019, 15, 1903180.
- Neelam, S.; Chancellor, T.J.; Li, Y.; Nickerson, J.A.; Roux, K.J.; Dickinson, R.B.; Lele, T.P. Direct Force Probe Reveals the Mechanics of Nuclear Homeostasis in the Mammalian Cell. Proc. Natl. Acad. Sci. USA 2015, 112, 5720–5725.
- Wei, J.; Xu, G.; Wu, M.; Zhang, Y.; Li, Q.; Liu, P.; Zhu, T.; Song, A.; Zhao, L.; Han, Z.; et al. Overexpression of Vimentin Contributes to Prostate Cancer Invasion and Metastasis via Src Regulation. Anticancer Res. 2008, 28, 327–334.
- Mendez, M.G.; Kojima, S.I.; Goldman, R.D. Vimentin Induces Changes in Cell Shape, Motility, and Adhesion during the Epithelial to Mesenchymal Transition. FASEB J. 2010, 24, 1838–1851.
- Zhu, Q.-S.; Rosenblatt, K.; Huang, K.-L.; Lahat, G.; Brobey, R.; Bolshakov, S.; Nguyen, T.; Ding, Z.; Belousov, R.; Bill, K.; et al. Vimentin Is a Novel AKT1 Target Mediating Motility and Invasion. Oncogene 2011, 30, 457–470.
- Vuoriluoto, K.; Haugen, H.; Kiviluoto, S.; Mpindi, J.-P.; Nevo, J.; Gjerdrum, C.; Tiron, C.; Lorens, J.B.; Ivaska, J. Vimentin Regulates EMT Induction by Slug and Oncogenic H-Ras and Migration by Governing Axl Expression in Breast Cancer. Oncogene 2011, 30, 1436–1448.
- Pawelzyk, P.; Mücke, N.; Herrmann, H.; Willenbacher, N. Attractive Interactions among Intermediate Filaments Determine Network Mechanics In Vitro. PLoS ONE 2014, 9, e93194.
- Lin, Y.-C.; Broedersz, C.P.; Rowat, A.C.; Wedig, T.; Herrmann, H.; MacKintosh, F.C.; Weitz, D.A. Divalent Cations Crosslink Vimentin Intermediate Filament Tail Domains to Regulate Network Mechanics. J. Mol. Biol. 2010, 399, 637–644.
- Bär, H.; Schopferer, M.; Sharma, S.; Hochstein, B.; Mücke, N.; Herrmann, H.; Willenbacher, N. Mutations in Desmin’s Carboxy-Terminal “Tail” Domain Severely Modify Filament and Network Mechanics. J. Mol. Biol. 2010, 397, 1188–1198.
- Bousquet, O.; Ma, L.; Yamada, S.; Gu, C.; Idei, T.; Takahashi, K.; Wirtz, D.; Coulombe, P.A. The Nonhelical Tail Domain of Keratin 14 Promotes Filament Bundling and Enhances the Mechanical Properties of Keratin Intermediate Filaments in Vitro. J. Cell Biol. 2001, 155, 747–754.
- Wagner, O.I.; Rammensee, S.; Korde, N.; Wen, Q.; Leterrier, J.-F.; Janmey, P.A. Softness, Strength and Self-Repair in Intermediate Filament Networks. Exp. Cell Res. 2007, 313, 2228–2235.
- Schopferer, M.; Bär, H.; Hochstein, B.; Sharma, S.; Mücke, N.; Herrmann, H.; Willenbacher, N. Desmin and Vimentin Intermediate Filament Networks: Their Viscoelastic Properties Investigated by Mechanical Rheometry. J. Mol. Biol. 2009, 388, 133–143.
- Janmey, P.A.; Euteneuer, U.; Traub, P.; Schliwa, M. Viscoelastic Properties of Vimentin Compared with Other Filamentous Biopolymer Networks. J. Cell Biol. 1991, 113, 155–160.
- Majumdar, S.; Foucard, L.C.; Levine, A.J.; Gardel, M.L. Mechanical Hysteresis in Actin Networks. Soft Matter 2018, 14, 2052–2058.
- Schmoller, K.M.; Fernández, P.; Arevalo, R.C.; Blair, D.L.; Bausch, A.R. Cyclic Hardening in Bundled Actin Networks. Nat. Commun. 2010, 1, 134.
- Aufderhorst-Roberts, A.; Koenderink, G.H. Stiffening and Inelastic Fluidization in Vimentin Intermediate Filament Networks. Soft Matter 2019, 15, 7127–7136.
- Pawelzyk, P.; Herrmann, H.; Willenbacher, N. Mechanics of Intermediate Filament Networks Assembled from Keratins K8 and K18. Soft Matter 2013, 9, 8871.
- Lin, Y.-C.; Yao, N.Y.; Broedersz, C.P.; Herrmann, H.; MacKintosh, F.C.; Weitz, D.A. Origins of Elasticity in Intermediate Filament Networks. Phys. Rev. Lett. 2010, 104, 058101.
- Yao, N.Y.; Broedersz, C.P.; Lin, Y.-C.; Kasza, K.E.; MacKintosh, F.C.; Weitz, D.A. Elasticity in Ionically Cross-Linked Neurofilament Networks. Biophys. J. 2010, 98, 2147–2153.
- Kreplak, L.; Bär, H.; Leterrier, J.F.; Herrmann, H.; Aebi, U. Exploring the Mechanical Behavior of Single Intermediate Filaments. J. Mol. Biol. 2005, 354, 569–577.
- Kreplak, L.; Herrmann, H.; Aebi, U. Tensile Properties of Single Desmin Intermediate Filaments. Biophys. J. 2008, 94, 2790–2799.
- Guzmán, C.; Jeney, S.; Kreplak, L.; Kasas, S.; Kulik, A.J.; Aebi, U.; Forró, L. Exploring the Mechanical Properties of Single Vimentin Intermediate Filaments by Atomic Force Microscopy. J. Mol. Biol. 2006, 360, 623–630.
- Staple, D.B.; Loparic, M.; Kreuzer, H.J.; Kreplak, L. Stretching, Unfolding, and Deforming Protein Filaments Adsorbed at Solid-Liquid Interfaces Using the Tip of an Atomic-Force Microscope. Phys. Rev. Lett. 2009, 102, 128302.
- Block, J.; Witt, H.; Candelli, A.; Peterman, E.J.G.; Wuite, G.J.L.; Janshoff, A.; Köster, S. Nonlinear Loading-Rate-Dependent Force Response of Individual Vimentin Intermediate Filaments to Applied Strain. Phys. Rev. Lett. 2017, 118, 048101.
- Pinto, N.; Yang, F.-C.; Negishi, A.; Rheinstädter, M.C.; Gillis, T.E.; Fudge, D.S. Self-Assembly Enhances the Strength of Fibers Made from Vimentin Intermediate Filament Proteins. Biomacromolecules 2014, 15, 574–581.
- Qin, Z.; Kreplak, L.; Buehler, M.J. Hierarchical Structure Controls Nanomechanical Properties of Vimentin Intermediate Filaments. PLoS ONE 2009, 4, e7294.
- Kreplak, L.; Fudge, D. Biomechanical Properties of Intermediate Filaments: From Tissues to Single Filaments and Back. Bioessays 2007, 29, 26–35.
- Kollmannsberger, P.; Fabry, B. Linear and Nonlinear Rheology of Living Cells. Annu. Rev. Mater. Res. 2011, 41, 75–97.
- Hol, E.M.; Pekny, M. Glial Fibrillary Acidic Protein (GFAP) and the Astrocyte Intermediate Filament System in Diseases of the Central Nervous System. Curr. Opin. Cell Biol. 2015, 32, 121–130.
- Cheah, J.S.; Jacobs, K.A.; Heinrich, V.; Lo, S.H.; Yamada, S. Force-Induced Recruitment of Cten along Keratin Network in Epithelial Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 19799–19801.




