
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elizabeth Barber | + 1217 word(s) | 1217 | 2021-08-27 04:57:57 | | | |
| 2 | Enzi Gong | Meta information modification | 1217 | 2021-08-31 02:32:10 | | | | |
| 3 | Dean Liu | Meta information modification | 1217 | 2021-09-28 06:06:00 | | |
Video Upload Options
Flavonoids are found ubiquitously in plants and represent ~60% of all dietary (poly)phenolic compounds. Flavonols, a sub-class of flavonoids, are present in onions, kale, apples, berries, leeks and broccoli. Some flavonols excreted in urine can be used as biomarkers of flavonol intake and are significantly associated with a lower T2D risk. Many flavonoids extracted from plants inhibit α-amylase and α-glucosidase activities in vitro, and improve postprandial glycaemia in diabetic animal models and limited human studies. Very few studies have reported on the inhibition of isomaltase, however. The disaccharide isomaltose is rarely present in nature but is commonly added as low-caloric food sweeteners in industrial-scale production, or produced from amylopectin hydrolysis to α-limit dextrins. Studies assessing the isomaltase inhibitory potential by flavonoids and acarbose are therefore of interest.
1. Overview
Certain flavonoids can influence glucose metabolism by inhibiting enzymes involved in carbohydrate digestion and suppressing intestinal glucose absorption. In this study, four structurally-related flavonols (quercetin, kaempferol, quercetagetin and galangin) were evaluated individually for their ability to inhibit human α-glucosidases (sucrase, maltase and isomaltase), and were compared with the antidiabetic drug acarbose and the flavan-3-ol (−)-epigallocatechin-3-gallate (EGCG). Cell-free extracts from human intestinal Caco-2/TC7 cells were used as the enzyme source and products were quantified chromatographically with high accuracy, precision and sensitivity. Acarbose inhibited sucrase, maltase and isomaltase with IC50 values of 1.65, 13.9 and 39.1 µM, respectively. A similar inhibition pattern, but with comparatively higher values, was observed with EGCG. Of the flavonols, quercetagetin was the strongest inhibitor of α-glucosidases, with inhibition constants approaching those of acarbose, followed by galangin and kaempferol, while the weakest were quercetin and EGCG. The varied inhibitory effects of flavonols against human α-glucosidases depend on their structures, the enzyme source and substrates employed. The flavonols were more effective than EGCG, but less so than acarbose, and so may be useful in regulating sugar digestion and postprandial glycaemia without the side effects associated with acarbose treatment.
2. Type 2 Diabetes
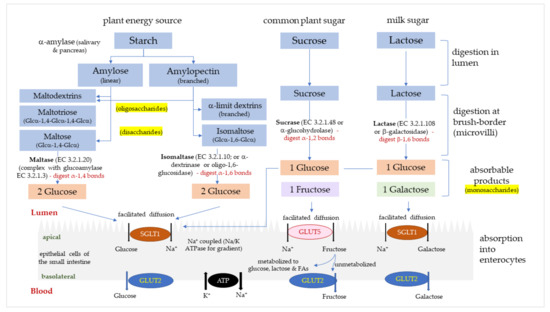
3. Conclusions
References
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63.
- Chiba, S. Molecular mechanism in alpha-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239.
- Rose, D.R.; Chaudet, M.M.; Jones, K. Structural Studies of the Intestinal alpha-Glucosidases, Maltase-glucoamylase and Sucrase-isomaltase. J. Pediatr. Gastroenterol. Nutr. 2018, 66 (Suppl. S3), S11–S13.
- Hamaker, B.R.; Lee, B.H.; Quezada-Calvillo, R. Starch digestion and patients with congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 2012, 55 (Suppl. S2), S24–S28.
- Dahlqvist, A. Specificity of the human intestinal disaccharidases and implications for hereditary disaccharide intolerance. J. Clin. Investig. 1962, 41, 463–470.
- Karnsakul, W.; Luginbuehl, U.; Hahn, D.; Sterchi, E.; Avery, S.; Sen, P.; Swallow, D.; Nichols, B. Disaccharidase activities in dyspeptic children: Biochemical and molecular investigations of maltase-glucoamylase activity. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 551–556.
- McIver, L.A.; Tripp, J. Acarbose. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Lin, W.H.; Yang, C.Y.; Kuo, S.; Kuo, T.H.; Roan, J.N.; Li, C.Y.; Wang, M.C.; Ou, H.T. Hepatic and cardiovascular safety of acarbose among type 2 diabetes patients with end-stage renal disease: A nationwide population-based longitudinal study. Diabetes Res. Clin. Pract. 2020, 108489.
- Li, C.; Kuang, J.; Zhao, Y.; Sun, H.; Guan, H. Effect of type 2 diabetes and antihyperglycemic drug therapy on signs of tumor invasion in papillary thyroid cancer. Endocrine 2020, 69, 92–99.
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2, e000327.
- Spiller, H.A. Management of antidiabetic medications in overdose. Drug Saf. 1998, 19, 411–424.
- Szymanska, R.; Pospisil, P.; Kruk, J. Plant-Derived Antioxidants in Disease Prevention 2018. Oxid. Med. Cell Longev. 2018, 2018, 2068370.
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17.
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam Repub. Iran 2017, 31, 134.
- Al-Duhaidahawi, D.; Hasan, S.A.; Al-Zubaidy, H.F.S. Flavonoids in the Treatment of Diabetes Clinical Outcomes and Mechanism to ameliorate Blood Glucose levels. Curr. Diabetes Rev. 2020.
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S.
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential alpha-amylase/alpha-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954.
- Woodward, K.A.; Draijer, R.; Thijssen, D.H.J.; Low, D.A. Polyphenols and Microvascular Function in Humans: A Systematic Review. Curr. Pharm. Des. 2018, 24, 203–226.
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as alpha-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836.
- Sun, Q.; Wedick, N.M.; Tworoger, S.S.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. Urinary Excretion of Select Dietary Polyphenol Metabolites Is Associated with a Lower Risk of Type 2 Diabetes in Proximate but Not Remote Follow-Up in a Prospective Investigation in 2 Cohorts of US Women. J. Nutr. 2015, 145, 1280–1288.
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal medicines for diabetes management and its secondary complications. Curr. Diabetes Rev. 2020.
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57.
- Van Beers, E.H.; Al, R.H.; Rings, E.H.H.M.; Einerhand, A.W.C.; Dekker, J.; Buller, H.A. Lactase and sucrase-isomaltase gene expression during Caco-2 cell differentiation. Biochem. J. 1995, 308, 769–775.
- Miyake, T.; Sakai, S.; Shibuya, T. Process for Producing a High-Purity Isomaltose. U.S. Patent 4,521,252, 26 October 1981.
- Turco, L.; Catone, T.; Caloni, F.; Di Consiglio, E.; Testai, E.; Stammati, A. Caco-2/TC7 cell line characterization for intestinal absorption: How reliable is this in vitro model for the prediction of the oral dose fraction absorbed in human? Toxicol. In Vitro 2011, 25, 13–20.
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of Human and Rat Sucrase and Maltase Activities To Assess Antiglycemic Potential: Optimization of the Assay Using Acarbose and Polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651.
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on alpha-amylase and alpha-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708.




