Flavonoids are found ubiquitously in plants and represent ~60% of all dietary (poly)phenolic compounds. Flavonols, a sub-class of flavonoids, are present in onions, kale, apples, berries, leeks and broccoli. Some flavonols excreted in urine can be used as biomarkers of flavonol intake and are significantly associated with a lower T2D risk. Many flavonoids extracted from plants inhibit α-amylase and α-glucosidase activities in vitro, and improve postprandial glycaemia in diabetic animal models and limited human studies. Very few studies have reported on the inhibition of isomaltase, however. The disaccharide isomaltose is rarely present in nature but is commonly added as low-caloric food sweeteners in industrial-scale production, or produced from amylopectin hydrolysis to α-limit dextrins. Studies assessing the isomaltase inhibitory potential by flavonoids and acarbose are therefore of interest.
1. Overview
Certain flavonoids can influence glucose metabolism by inhibiting enzymes involved in carbohydrate digestion and suppressing intestinal glucose absorption. In this study, four structurally-related flavonols (quercetin, kaempferol, quercetagetin and galangin) were evaluated individually for their ability to inhibit human α-glucosidases (sucrase, maltase and isomaltase), and were compared with the antidiabetic drug acarbose and the flavan-3-ol (−)-epigallocatechin-3-gallate (EGCG). Cell-free extracts from human intestinal Caco-2/TC7 cells were used as the enzyme source and products were quantified chromatographically with high accuracy, precision and sensitivity. Acarbose inhibited sucrase, maltase and isomaltase with IC50 values of 1.65, 13.9 and 39.1 µM, respectively. A similar inhibition pattern, but with comparatively higher values, was observed with EGCG. Of the flavonols, quercetagetin was the strongest inhibitor of α-glucosidases, with inhibition constants approaching those of acarbose, followed by galangin and kaempferol, while the weakest were quercetin and EGCG. The varied inhibitory effects of flavonols against human α-glucosidases depend on their structures, the enzyme source and substrates employed. The flavonols were more effective than EGCG, but less so than acarbose, and so may be useful in regulating sugar digestion and postprandial glycaemia without the side effects associated with acarbose treatment.
2. Type 2 Diabetes
One of the earliest signs of type 2 diabetes (T2D) is elevated and erratic postprandial glycaemia that promotes oxidative stress at various sites within the body
[1]. Controlling postprandial glycaemia is an important strategy in the management of T2D. One way is by slowing down carbohydrate digestion and glucose absorption in the intestine via the inhibition of salivary/pancreatic α-amylases and membrane-bound brush-border α-glucosidases.
There are four relevant digestive α-glucosidases in humans, maltase (α-1,4-glucosidase; EC 3.2.1.20), glucoamylase (exo-1,4-α-glucosidase; EC 3.2.1.3), sucrase (α-glucohydrolase; EC 3.2.1.48) and isomaltase (oligo-1,6-glucosidase or α-dextrinase; EC 3.2.1.10). Maltase and glucoamylase have a unique, high α-1,4 hydrolytic activity for longer chain maltooligosaccharides to produce glucose
[2], and are referred to as maltase/glucoamylase (MGAM)
[3]. Sucrase-isomaltase (SI) is synthesized as a single glycoprotein chain in intestinal cells
[4], and then cleaved into individual sucrase and isomaltase domains that reassociate non-covalently. Sucrase hydrolyses α-1,2-glycosidic bonds in sucrose to produce glucose and fructose. Isomaltase is the only enzyme able to hydrolyze the α-1,6-glycosidic linkage in α-limit dextrins to produce glucose. MGAM and SI complexes are located along the entire small intestine
[5][6][5,6] and function to catalyze the production of glucose and fructose from disaccharides, dextrins and dietary polysaccharides. Glucose and fructose pass across intestinal cell membranes via glucose transporters (GLUTs), mainly sodium-glucose transport protein-1 (SGLT1) and glucose transporters -2 and -5 (GLUT2, GLUT5). The pathway of carbohydrate hydrolysis and absorption in the intestine is summarized in
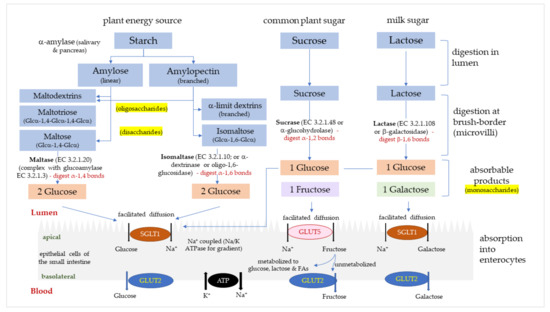
Figure 1. Rapidly digested and absorbed glucose in the intestine results in a sharp increase in plasma glucose, which is regulated by insulin-stimulated uptake of glucose into tissues.
Figure 1. The digestion and absorption of starch and sugar in the small intestine. Glucose, fructose and galactose are absorbed into enterocytes via glucose transporters (GLUTs); sodium-glucose transport protein-1 (SGLT1) and glucose transporters -2 and -5 (GLUT2, GLUT5).
The most commonly used FDA-approved pharmaceutical α-glucosidase inhibitor is acarbose, a fermented product from
Actinoplanes species
[7] that is low-risk and non-toxic
[8][9][8,9], but is associated with uncomfortable side effects such as bloating, cramping, flatulence and abdominal pain
[10], and drug-intolerance with chronic treatment
[11]. Several potent α-glucosidase inhibitors from plant sources have been identified and have received great attention from the scientific community worldwide as they possess no evident side effects
[12][13][12,13]. Among them were flavonoids, the most extensively studied compounds as natural antidiabetic agents, associated with a reduction in risk of diabetes in humans, animals and in vitro models
[14][15][14,15]. Food-derived flavonoids show extremely low toxicity
[16][17][18][16,17,18].
Flavonoids are found ubiquitously in plants and represent ~60% of all dietary (poly)phenolic compounds
[19][20][19,20]. Flavonols, a sub-class of flavonoids, are present in onions, kale, apples, berries, leeks and broccoli
[19]. Some flavonols excreted in urine can be used as biomarkers of flavonol intake and are significantly associated with a lower T2D risk
[21]. Many flavonoids extracted from plants inhibit α-amylase and α-glucosidase activities in vitro, and improve postprandial glycaemia in diabetic animal models and limited human studies
[22][23][22,23]. Very few studies have reported on the inhibition of isomaltase, however. The disaccharide isomaltose is rarely present in nature but is commonly added as low-caloric food sweeteners in industrial-scale production
[24][25][24,25], or produced from amylopectin hydrolysis to α-limit dextrins. Studies assessing the isomaltase inhibitory potential by flavonoids and acarbose are therefore of interest.
Unfortunately, many enzyme inhibition studies have been conducted using α-glucosidases from yeast or bacteria, with fewer studies using human intestinal enzymes. The inhibition of yeast and human α-glucosidases is very different, specific to the type of substrate, as reported for maltose
[2]. Here we used Caco-2 cells, originating from human colon cancer cells, which form monolayers that differentiate to produce apical microvilli with high expression of maltase and sucrase. The Caco-2/TC7 clone specifically expresses high SI levels at 19–25 days post-confluence
[24][26][24,26]. Using an enzyme preparation from these cells, we have evaluated sucrase, maltase and isomaltase inhibition by several flavonols and compared them to acarbose and (−)-epigallocatechin-3-gallate (EGCG), a flavan-3-ol known for its inhibitory activity on sucrase and maltase of various sources
[27]. These natural compounds may provide promising alternatives for diabetes management with no undesirable side effects.
3. Conclusions
A sensitive and accurate method to determine sugar hydrolysis by sucrase, maltase and isomaltase has been successfully developed and validated. The use of HPAE-PAD, a type of ion chromatography specialized for the analysis of carbohydrates, to detect subtle changes in the concentrations of five sugars simultaneously, with minimal sample preparation and high precision within 32 min, has been central to this study. Acarbose and flavonoids exhibit different inhibition of human enzymes to those reported for yeast or mammalian α-glucosidases, emphasizing the need for a more pragmatic screening approach on individual human enzymes to elucidate their actual inhibitory potentials in vivo. Flavonoids from various sources are more effective against α-glucosidases than α-amylase
[28][37]. The low solubility of some flavonoids limits the experimental concentration which can be employed, preventing the determination of IC
50 values and necessitating the use of IC
25 or IC
15 values instead.
Quercetagetin, similar to acarbose, followed by kaempferol and galangin, exhibited greater inhibitory action against sucrase, maltase and isomaltase than EGCG and quercetin, although the latter compounds were more soluble in aqueous buffer. Two key structural elements of flavonoids for enhanced α-glucosidase inhibition in humans are the C
6-OH A ring hydroxylation and reduced B ring hydroxylation. Improving understanding of how flavonoids bind to human α-glucosidases should provide a rational basis for exploiting antidiabetic compounds from dietary sources.