
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benyamin Rosental | + 2046 word(s) | 2046 | 2021-08-26 10:47:17 | | | |
| 2 | Vicky Zhou | -87 word(s) | 1959 | 2021-08-30 07:50:06 | | | | |
| 3 | Edna Ayerim Mandujano-Tinoco | + 26 word(s) | 1985 | 2021-08-30 22:35:26 | | |
Video Upload Options
Botryllus schlosseri, a colonial tunicate, which is the nearest invertebrate group to the vertebrates, is devoid of T- and B-cell-based adaptive immunity. It has unique characteristics that make it a valuable model system for studying innate immunity mechanisms: (i) a natural allogeneic transplantation phenomenon that results in either fusion or rejection; (ii) whole animal regeneration and noninflammatory resorption on a weekly basis; (iii) allogeneic resorption which is comparable to human chronic rejection. Recent studies in B. schlosseri have led to the recognition of a molecular and cellular framework underlying the innate immunity loss of tolerance to allogeneic tissues. Additionally, B. schlosseri was developed as a model for studying hematopoietic stem cell (HSC) transplantation, and it provides further insights into the similarities between the HSC niches of human and B. schlosseri.
The entry is from Botryllus schlosseri as a Unique Colonial Chordate Model for the Study and Modulation of Innate Immune Activity. August 2021Marine Drugs 19(8):454. DOI: 10.3390/md19080454
1. Introduction
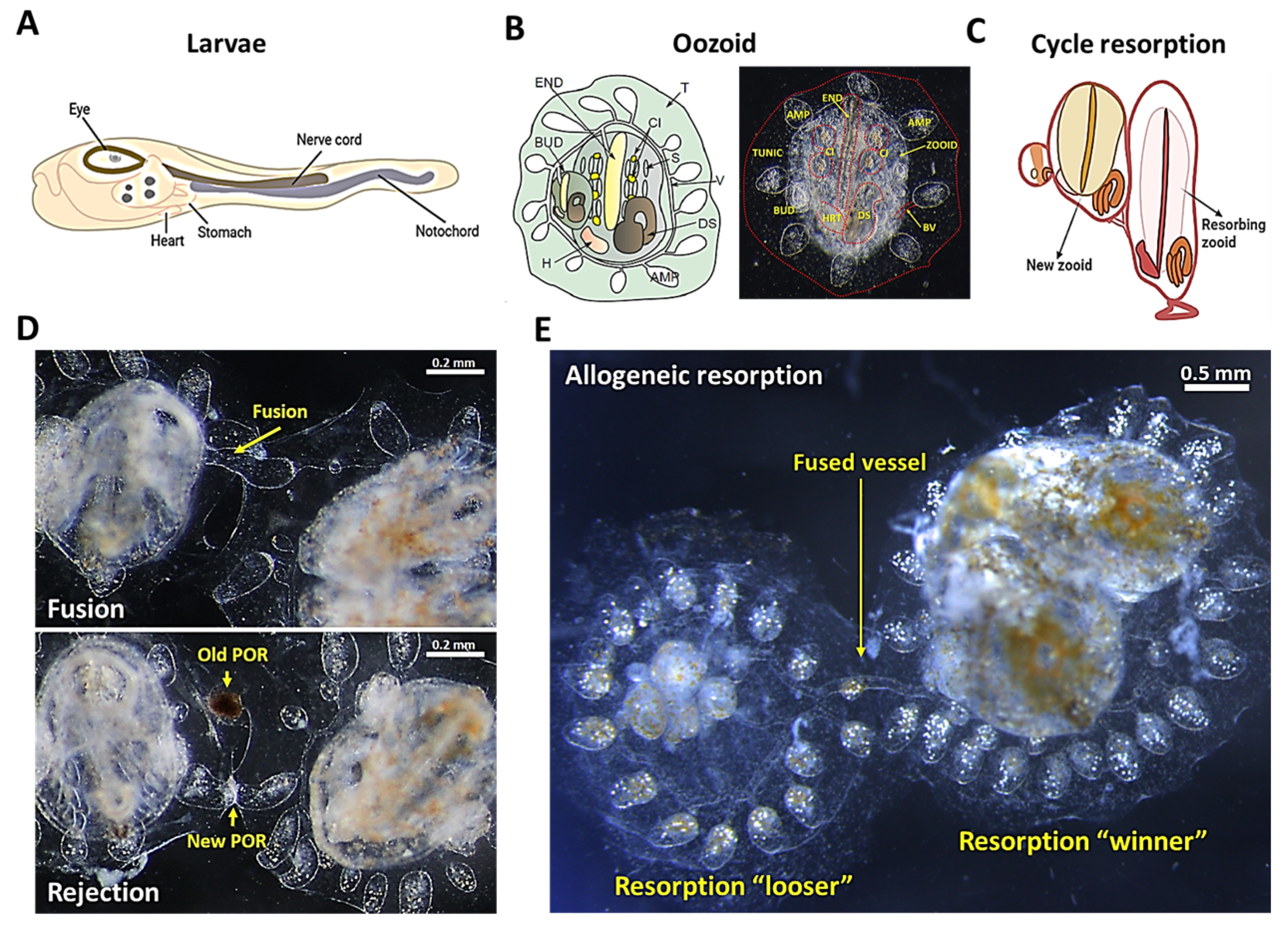
2. Botryllus schlosseri as a Model to Study the Innate Immunity
3. Conclusions
References
- Ben-Shlomo, R.; Reem, E.; Douek, J.; Rinkevich, B. Population genetics of the invasive ascidian Botryllus schlosseri from South American coasts. Mar. Ecol. Prog. Ser. 2010, 412, 85.
- Stoner, D.S.; Ben-Shlomo, R.; Rinkevich, B.; Weissman, I.L. Genetic variability of Botryllus schlosseri invasions to the east and west coasts of the USA. Mar. Ecol. Prog. Ser. 2002, 243, 93.
- Darwin, C.; Kebler, L. On the Origin of Sspecies by Means of Natural Selection, or, The Preservation of Favoured Races in the Struggle for Life; J. Murray: London, UK, 1859; Volume 1, p. 502.
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965.
- Manni, L.; Lane, N.J.; Joly, J.S.; Gasparini, F.; Tiozzo, S.; Caicci, F.; Zaniolo, G.; Burighel, P. Neurogenic and non-neurogenic placodes in ascidians. J. Exp. Zoology. Part B Mol. Dev. Evol. 2004, 302, 483.
- Kowarsky, M.; Anselmi, C.; Hotta, K.; Burighel, P.; Zaniolo, G.; Caicci, F.; Rosental, B.; Neff, N.F.; Ishizuka, K.J.; Palmeri, K.J.; et al. Sexual and asexual development: Two distinct programs producing the same tunicate. Cell Rep. 2021, 34, 108681.
- Manni, L.; Gasparini, F.; Hotta, K.; Ishizuka, K.J.; Ricci, L.; Tiozzo, S.; Voskoboynik, A.; Dauga, D. Ontology for the Asexual Development and Anatomy of the Colonial Chordate Botryllus schlosseri. PLoS ONE 2014, 9, e96434.
- Voskoboynik, A.; Neff, N.F.; Sahoo, D.; Newman, A.M.; Pushkarev, D.; Koh, W.; Passarelli, B.; Fan, H.C.; Mantalas, G.L.; Palmeri, K.J.; et al. The genome sequence of the colonial chordate, Botryllus schlosseri. eLife 2013, 2, e00569.
- Manni, L.; Burighel, P. Common and divergent pathways in alternative developmental processes of ascidians. BioEssays 2006, 28, 902–912.
- Milkman, R. Genetic and developmental studies on Botryllus schlosseri. Biol. Bull. 1967, 132, 229–243.
- Rosental, B.; Kowarsky, M.; Seita, J.; Corey, D.M.; Ishizuka, K.J.; Palmeri, K.J.; Chen, S.-Y.; Sinha, R.; Okamoto, J.; Mantalas, G.; et al. Complex mammalian-like haematopoietic system found in a colonial chordate. Nature 2018, 564, 425.
- Cima, F.; Basso, G.; Ballarin, L. Apoptosis and phosphatidylserine-mediated recognition during the take-over phase of the colonial life-cycle in the ascidian Botryllus schlosseri. Cell Tissue Res. 2003, 312, 369–376.
- Lauzon, R.J.; Ishizuka, K.J.; Weissman, I.L. A cyclical, developmentally-regulated death phenomenon in a colonial urochordate. Dev. Dyn. 1992, 194, 71–83.
- Lauzon, R.J.; Patton, C.W.; Weissman, I.L. A morphological and immunohistochemical study of programmed cell death in Botryllus schlosseri (Tunicata, Ascidiacea). Cell Tissue Res. 1993, 272, 115–127.
- Laird, D.J.; De Tomaso, A.W.; Cooper, M.D.; Weissman, I.L. 50 million years of chordate evolution: Seeking the origins of adaptive immunity. Proc. Natl. Acad. Sci. USA 2000, 97, 6924–6926.
- Laird, D.J.; De Tomaso, A.W.; Weissman, I.L. Stem Cells are Units of Natural Selection in a Colonial Ascidian. Cell 2005, 123, 1351–1360.
- Rinkevich, Y.; Voskoboynik, A.; Rosner, A.; Rabinowitz, C.; Paz, G.; Oren, M.; Douek, J.; Alfassi, G.; Moiseeva, E.; Ishizuka, K.J.; et al. Repeated, Long-Term Cycling of Putative Stem Cells between Niches in a Basal Chordate. Dev. Cell 2013, 24, 76–88.
- Stoner, D.S.; Rinkevich, B.; Weissman, I.L. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc. Natl. Acad. Sci. USA 1999, 96, 9148–9153.
- Stoner, D.S.; Weissman, I.L. Somatic and germ cell parasitism in a colonial ascidian: Possible role for a highly polymorphic allorecognition system. Proc. Natl. Acad. Sci. USA 1996, 93, 15254–15259.
- Voskoboynik, A.; Soen, Y.; Rinkevich, Y.; Rosner, A.; Ueno, H.; Reshef, R.; Ishizuka, K.J.; Palmeri, K.J.; Moiseeva, E.; Rinkevich, B.; et al. Identification of the Endostyle as a Stem Cell Niche in a Colonial Chordate. Cell Stem Cell 2008, 3, 456–464.
- Corey, D.M.; Rosental, B.; Kowarsky, M.; Sinha, R.; Ishizuka, K.J.; Palmeri, K.J.; Quake, S.R.; Voskoboynik, A.; Weissman, I.L. Developmental cell death programs license cytotoxic cells to eliminate histocompatible partners. Proc. Natl. Acad. Sci. USA 2016, 113, 6520–6525.
- Sabbadin, A. Le basi genetiche della capacita di fusione fra colonie in Botryllus schlosseri (Ascidiacea). Rend Accad Naz Lincei Ser VIII 1962, 32, 1031–1035.
- Scofield, V. Allorecognition and microbial infection: Roles in the evolution of sex and immunity. In The Origin and Evolution of Sex; Halvorson, H., Monroy, A., Eds.; Alan R. Liss: New York, NY, USA, 1985; Volume 7, p. 213.
- Voskoboynik, A.; Newman, A.M.; Corey, D.M.; Sahoo, D.; Pushkarev, D.; Neff, N.F.; Passarelli, B.; Koh, W.; Ishizuka, K.J.; Palmeri, K.J.; et al. Identification of a Colonial Chordate Histocompatibility Gene. Science 2013, 341, 384.
- Rinkevich, B.; Tartakover, S.; Gershon, H. Contribution of morula cells to allogeneic responses in the colonial urochordate Botryllus schlosseri. Mar. Biol. 1998, 131, 227–236.
- Ueno, H.; Weissman, I.L. Clonal Analysis of Mouse Development Reveals a Polyclonal Origin for Yolk Sac Blood Islands. Dev. Cell 2006, 11, 519.
- Ueno, H.; Turnbull, B.B.; Weissman, I.L. Two-step oligoclonal development of male germ cells. Proc. Natl. Acad. Sci. USA 2009, 106, 175–180.
- Weissman, I.L. Stem cells are units of natural selection for tissue formation, for germline development, and in cancer development. Proc. Natl. Acad. Sci. USA 2015, 112, 8922–8928.
- Beerman, I.; Bhattacharya, D.; Zandi, S.; Sigvardsson, M.; Weissman, I.L.; Bryder, D.; Rossi, D.J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 5465–5470.
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017.
- Rossi, D.J.; Bryder, D.; Seita, J.; Nussenzweig, A.; Hoeijmakers, J.; Weissman, I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nat. Cell Biol. 2007, 447, 725–729.
- Rossi, D.J.; Bryder, D.; Zahn, J.M.; Ahlenius, H.; Sonu, R.; Wagers, A.J.; Weissman, I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 2005, 102, 9194–9199.
- Rossi, D.J.; Jamieson, C.H.; Weissman, I.L. Stems Cells and the Pathways to Aging and Cancer. Cell 2008, 132, 681–696.
- Corces, M.; Hong, W.-J.; Weissman, I.L.; Medeiros, B.C.; Majeti, R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc. Natl. Acad. Sci. USA 2014, 111, 2548–2553.
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; Van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285.
- Jamieson, C.H.; Ailles, L.E.; Dylla, S.J.; Muijtjens, M.; Jones, C.; Zehnder, J.L.; Gotlib, J.; Li, K.; Manz, M.G.; Keating, A.; et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 2004, 351, 657–667.
- Jan, M.; Snyder, T.M.; Corces-Zimmerman, M.R.; Vyas, P.; Weissman, I.L.; Quake, S.R.; Majeti, R. Clonal Evolution of Preleukemic Hematopoietic Stem Cells Precedes Human Acute Myeloid Leukemia. Sci. Transl. Med. 2012, 4, 149ra118.
- Miyamoto, T.; Weissman, I.L.; Akashi, K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl. Acad. Sci. USA 2000, 97, 7521–7526.
- Sykes, S.M.; Kokkaliaris, K.D.; Milsom, M.D.; Levine, R.L.; Majeti, R. Clonal evolution of preleukemic hematopoietic stem cells in acute myeloid leukemia. Exp. Hematol. 2015, 43, 989–992.
- Kojima, Y.; Volkmer, J.-P.; McKenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; Direnzo, D.; Nanda, V.; Ye, J.; Connolly, A.J.; et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016, 536, 86.
- Wang, B.; Zhang, F.; Wang, S.; Yang, R.; Chen, C.; Zhao, W. Imaging endogenous HClO in atherosclerosis using a novel fast-response fluorescence probe. Chem. Commun. 2020, 56, 2598.
- Rosental, B.; Raveh, T.; Voskoboynik, A.; Weissman, I.L. Evolutionary perspective on the hematopoietic system through a colonial chordate: Allogeneic immunity and hematopoiesis. Curr. Opin. Immunol. 2020, 62, 91.
- Franchi, N.; Ballarin, L. Immunity in Protochordates: The Tunicate Perspective. Front. Immunol. 2017, 8, 674.
- Lauzon, R.J.; Brown, C.; Kerr, L.; Tiozzo, S. Phagocyte dynamics in a highly regenerative urochordate: Insights into development and host defense. Dev. Biol. 2013, 374, 357–373.
- Peronato, A.; Franchi, N.; Loriano, B. BsTLR1: A new member of the TLR family of recognition proteins from the colonial ascidian Botryllus schlosseri. Fish Shellfish. Immunol. 2020, 106, 967.
- Khalturin, K.; Becker, M.; Rinkevich, B.; Bosch, T.C. Urochordates and the origin of natural killer cells: Identification of a CD94/NKR-P1-related receptor in blood cells of Botryllus. Proc. Natl. Acad. Sci. USA 2003, 100, 622–627.
- Franchi, N.; Schiavon, F.; Carletto, M.; Gasparini, F.; Bertoloni, G.; Tosatto, S.C.; Ballarin, L. Immune roles of a rhamnose-binding lectin in the colonial ascidian Botryllus schlosseri. Immunobiolgy 2011, 216, 725–736.
- Franchi, N.; Schiavon, F.; Betti, M.; Canesi, L.; Ballarin, L. Insight on signal transduction pathways involved in phagocytosis in the colonial ascidian Botryllus schlosseri. J. Invertebr. Pathol. 2013, 112, 260–266.
- Ballarin, L. Immunobiology of compound ascidians, with particular reference to Botryllus schlosseri: State of art. Invertebr. Surviv. J. 2008, 5, 54.
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755.
- Franchi, N.; Ballarin, L. Preliminary characterization of complement in a colonial tunicate: C3, Bf and inhibition of C3 opsonic activity by compstatin. Dev. Comp. Immunol. 2014, 46, 430.
- Peronato, A.; Drago, L.; Rothbacher, U.; Macor, P.; Ballarin, L.; Franchi, N. Complement system and phagocytosis in a colonial protochordate. Dev. Comp. Immunol. 2020, 103, 103530.
- Nicola, F.; Loriano, B. Morula cells as key hemocytes of the lectin pathway of complement activation in the colonial tunicate Botryllus schlosseri. Fish Shellfish. Immunol. 2017, 63, 157.
- Ballarin, L.; Menin, A.; Franchi, N.; Bertoloni, G.; Cima, F. Morula cells and non-self recognition in the compound ascidian Botryllus schlosseri. Invertebr. Surviv. J. 2005, 2, 1.
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development. Nat. Prod. Bioprosp. 2017, 7, 1–111.
- Ramesh, C.; Tulasi, B.R.; Raju, M.; Thakur, N.; Dufosse, L. Marine Natural Products from Tunicates and Their Associated Microbes. Mar. Drugs 2021, 19, 308.
- De Tomaso, A.W.; Nyholm, S.V.; Palmeri, K.J.; Ishizuka, K.J.; Ludington, W.B.; Mitchel, K.; Weissman, I.L. Isolation and characterization of a protochordate histocompatibility locus. Nature 2005, 438, 454.





