
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Niels Cremers | + 2388 word(s) | 2388 | 2021-08-26 10:22:32 | | | |
| 2 | Lindsay Dong | Meta information modification | 2388 | 2021-08-27 10:16:25 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2388 | 2021-08-30 08:02:13 | | |
Video Upload Options
Recurrent vulvovaginal candidiasis (RVVC) is a relapsing vaginal fungal infection caused by Candida species. In 57% of the cases, relapses occur within six months after fluconazole maintenance therapy, which is the current standard of care. The pathogenesis of RVVC is multifactorial, and recent studies have demonstrated that the vaginal microenvironment and activity of the immune system have a strong influence on the disease.
1. Introduction
2. Diagnosis of RVVC
3. Pathogenesis of RVVC
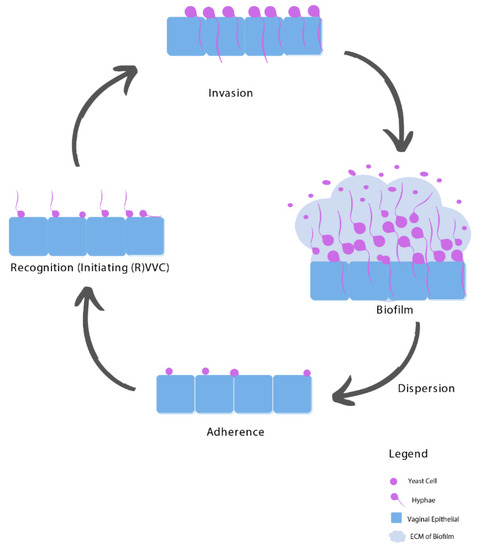
3.1. Adhesion
3.2. Recognition
3.3. Invasion
3.4. Biofilms
4. Risk Factors of RVVC
4.1. Imbalanced Vaginal Microbiota Composition
4.2. Host-Related Predisposing Factors
4.3. Idiopathic RVVC
5. Treatment of RVVC
5.1. Resistance towards Fluconazole
5.2. Unnecessary and Inappropriate Use of Fluconazole
5.3. Non-Albicans Species
5.4. Biofilms Complicate RVVC Treatment
6. Medical-Grade Honey as an Alternative Treatment Option
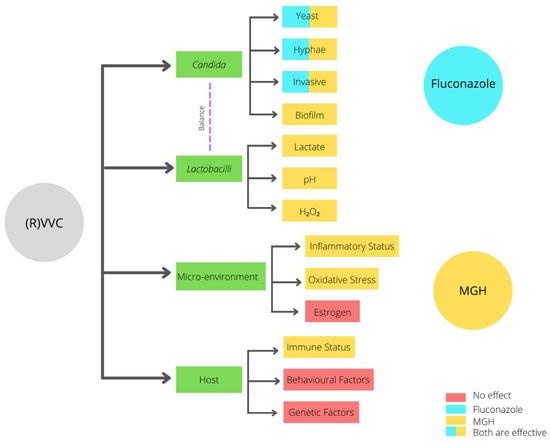
The high recurrence rate of complaints after fluconazole treatment may be attributed to the fact that fluconazole only interacts with the yeast, hyphae, and invasive Candida stages (Figure 2). In contrast, when an established biofilm is present, the ECM prevents the fluconazole from reaching the Candida cells, and therefore it will not have an effect on biofilms [31]. Moreover, fluconazole does not affect the vaginal mucosal response [1][3][4]. Since ancient times, honey has been used for wound treatment and care because of its antimicrobial and wound healing activities. Acquired azole resistance, the epidemiological shift from Candida albicans to NAC species, and the existence of biofilms demand better treatment options. Medical-grade honey (MGH) could be an accessible, effective, and affordable option [23]. To assure the safety and efficacy of honey for clinical application, strict guidelines are followed to establish MGH [32]. MGH is effective in acute and chronic wounds and provides rapid epithelization and wound contraction, has anti-inflammatory activity, stimulates debridement, decreases pain, resolves infections, decreases wound healing time, and is cost-effective [33]. The use of honey for reducing biofilm formation on indwelling plastic devices such as urinary catheters are also considered, but more research is needed [34][35].
| Characteristic | Fluconazole | MGH |
|---|---|---|
| Candida albicans | + | + |
| (Increased raise in VVC caused by) NAC species | − | + |
| Biofilms | − | + |
| Increased resistance | − | + |
| Microenvironment/vaginal mucosal response | − | + |
| Lactobacilli | − | +− |
| pH | − | + |
| Osmotic effect | − | + |
| Antimicrobial | + | + |
| Anti-inflammatory | − | + |
| Antioxidative | − | + |
Detailed mechanisms of how MGH affects the indicated pathways are described in the original manuscript. Extensive pre-clinical and clinical literature is discussed that supports that MGH is a promising treatment for RVVC. In addition, a new randomized-controlled trial (clinicaltrials.gov NCT04626258) is presented that intents to investigate the mycological and clinical cure and the prophylactic efficacy of the MGH-formulation L-Mesitran Soft in relation to the standard of care (fluconazole). L-Mesitran Soft is CE- and FDA approved and contains 40% MGH and different supplements that consistently have demonstrated to enhance the antimicrobial and wound healing activities.
7. Conclusions
References
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21.
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347.
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C., 3rd; Horowitz, B.; Von Thron, J.; et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883.
- Rosati, D.; Bruno, M.; Jaeger, M.; Ten Oever, J.; Netea, M.G. Recurrent Vulvovaginal Candidiasis: An Immunological Perspective. Microorganisms 2020, 8, 144.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Bauters, T.G.; Dhont, M.A.; Temmerman, M.I.; Nelis, H.J. Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am. J. Obstet. Gynecol. 2002, 187, 569–574.
- Banaeian-Borujeni, S.; Mobini, G.R.; Pourgheysari, B.; Validi, M. Comparison of the effect of honey and miconazole against Candida albicans in vitro. Adv. Biomed. Res. 2013, 2, 57.
- Gardella, C.E.L.; Lentz, G.M. Comprehensive Gynecology; Lentz, G.L.R., Gershenson, D., Valea, F.A., Eds.; Elsevier: Philadelphia, PA, USA, 2012; Volume 7, pp. 524–565.
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2015, 64, 1–137.
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.J.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211.
- Lema, V.M. Recurrent Vulvo-Vaginal Candidiasis: Diagnostic and Management Challenges in a Developing Country Context. Obstet. Gynecol. Int. J. 2017, 7, 260.
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding, C.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246.
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4.
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346.
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5.
- Cauchie, M.; Desmet, S.; Lagrou, K. Candida and its dual lifestyle as a commensal and a pathogen. Res. Microbiol. 2017, 168, 802–810.
- Wachtler, B.; Citiulo, F.; Jablonowski, N.; Forster, S.; Dalle, F.; Schaller, M.; Wilson, D.; Hube, B. Candida albicans-epithelial interactions: Dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS ONE 2012, 7, e36952.
- Peters, B.M.; Palmer, G.E.; Nash, A.K.; Lilly, E.A.; Fidel, P.L., Jr.; Noverr, M.C. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014, 82, 532–543.
- Re, A.C.S.; Martins, J.F.; Cunha-Filho, M.; Gelfuso, G.M.; Aires, C.P.; Gratieri, T. New perspectives on the topical management of recurrent candidiasis. Drug Deliv. Transl. Res. 2021, 11, 1568–1585.
- Sherry, L.; Kean, R.; McKloud, E.; O’Donnell, L.E.; Metcalfe, R.; Jones, B.L.; Ramage, G. Biofilms Formed by Isolates from Recurrent Vulvovaginal Candidiasis Patients Are Heterogeneous and Insensitive to Fluconazole. Antimicrob. Agents Chemother. 2017, 61, e01065-17.
- Muzny, C.A.; Schwebke, J.R. Biofilms: An Underappreciated Mechanism of Treatment Failure and Recurrence in Vaginal Infections. Clin. Infect. Dis. 2015, 61, 601–606.
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321.
- Zangl, I.; Pap, I.J.; Aspock, C.; Schuller, C. The role of Lactobacillus species in the control of Candida via biotrophic interactions. Microb. Cell 2019, 7, 1–14.
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376.
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245.
- Marchaim, D.; Lemanek, L.; Bheemreddy, S.; Kaye, K.S.; Sobel, J.D. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 2012, 120, 1407–1414.
- Makanjuola, O.; Bongomin, F.; Fayemiwo, S.A. An Update on the Roles of Non-albicans Candida Species in Vulvovaginitis. J. Fungi 2018, 4, 121.
- Jackson, S.T.; Mullings, A.M.; Rainford, L.; Miller, A. The epidemiology of mycotic vulvovaginitis and the use of antifungal agents in suspected mycotic vulvovaginitis and its implications for clinical practice. West. Indian Med. J. 2005, 54, 192–195.
- Rodriguez-Cerdeira, C.; Martinez-Herrera, E.; Carnero-Gregorio, M.; Lopez-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; Gonzalez-Cespon, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480.
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337.
- Vediyappan, G.; Rossignol, T.; d’Enfert, C. Interaction of Candida albicans biofilms with antifungals: Transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 2010, 54, 2096–2111.
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135.
- Yilmaz, A.C.; Aygin, D. Honey Dressing in Wound Treatment: A Systematic Review. Complement. Ther. Med. 2020, 51, 102388.
- Emineke, S.; Cooper, A.J.; Fouch, S.; Birch, B.R.; Lwaleed, B.A. Diluted honey inhibits biofilm formation: Potential application in urinary catheter management? J. Clin. Pathol. 2017, 70, 140–144.
- Aissat, S.; Ahmed, M.; Djebli, N. Propolis-Sahara honeys preparation exhibits antibacterial and anti-biofilm activity against bacterial biofilms formed on urinary catheters. Asian Pac. J. Trop. Dis. 2016, 6, 873–877.




