Recurrent vulvovaginal candidiasis (RVVC) is a relapsing vaginal fungal infection caused by Candida species. In 57% of the cases, relapses occur within six months after fluconazole maintenance therapy, which is the current standard of care. The pathogenesis of RVVC is multifactorial, and recent studies have demonstrated that the vaginal microenvironment and activity of the immune system have a strong influence on the disease.
- recurrent vulvovaginal candidiasis
- medical-grade honey
- fluconazole
- alternative treatment
- micro-environment modulation
1. Introduction
2. Diagnosis of RVVC
3. Pathogenesis of RVVC
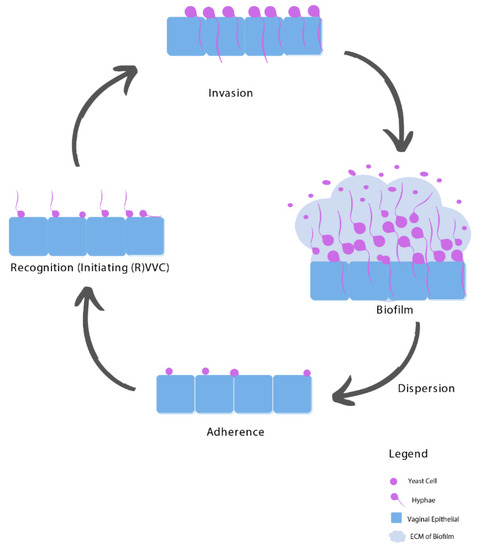
3.1. Adhesion
3.2. Recognition
3.3. Invasion
3.4. Biofilms
4. Risk Factors of RVVC
4.1. Imbalanced Vaginal Microbiota Composition
Alteration in the mucosal ecosystem leading to fungal dysbiosis can lead to (R)VVC and its symptoms [4]. A healthy vaginal microflora consists of different microorganisms, mainly Lactobacilli, but also accommodating fungi such as Candida albicans and Candida glabrata, living in symbiosis. Lactobacilli species play an important role in maintaining a healthy vaginal microbiome [4]. Through their presence, Lactobacilli species decrease opportunism of potentially pathogenic microorganisms by microbial competition, which reduces the adherence of Candida species to the vaginal epithelium [23][24][24,39].4.2. Host-Related Predisposing Factors
4.3. Idiopathic RVVC
5. Treatment of RVVC
5.1. Resistance towards Fluconazole
Since fluconazole is fungistatic rather than fungicidal, there is an increased opportunity to develop acquired resistance in the presence of this antifungal [25][46]. There are several challenges in fluconazole treatment such as an increase in antifungal resistance and VVC caused by NAC species, as well as the existence of biofilms [26][48]. Epidemiologic studies confirm that mostly all women diagnosed with fluconazole-resistant Candida albicans were previously exposed to fluconazole [26][48].5.2. Unnecessary and Inappropriate Use of Fluconazole
5.3. Non-Albicans Species
5.4. Biofilms Complicate RVVC Treatment
The biofilm formation in the pathogenesis of VVC provides elevated virulence and resistance towards antifungal therapy such as fluconazole [15][20][21][27,32,33]. A 1000-fold higher resistance profile of biofilms compared to their planktonic counterparts has been described [16][29][30][20,55,56]. Biofilms are also less sensitive to eradicate by the host immune system. A study of clinical isolates obtained from women with at least two episodes of VVC confirmed a lower antifungal susceptibility for biofilms in comparison with the planktonic antifungal susceptibility [20][32].6. Medical-Grade Honey as an Alternative Treatment Option
The high recurrence rate of complaints after fluconazole treatment may be attributed to the fact that fluconazole only interacts with the yeast, hyphae, and invasive Candida stages (Figure 2). In contrast, when an established biofilm is present, the ECM prevents the fluconazole from reaching the Candida cells, and therefore it will not have an effect on biofilms [31][57]. Moreover, fluconazole does not affect the vaginal mucosal response [1][3][4][1,3,4]. Since ancient times, honey has been used for wound treatment and care because of its antimicrobial and wound healing activities. Acquired azole resistance, the epidemiological shift from Candida albicans to NAC species, and the existence of biofilms demand better treatment options. Medical-grade honey (MGH) could be an accessible, effective, and affordable option [23][24]. To assure the safety and efficacy of honey for clinical application, strict guidelines are followed to establish MGH [32][58]. MGH is effective in acute and chronic wounds and provides rapid epithelization and wound contraction, has anti-inflammatory activity, stimulates debridement, decreases pain, resolves infections, decreases wound healing time, and is cost-effective [33][59]. The use of honey for reducing biofilm formation on indwelling plastic devices such as urinary catheters are also considered, but more research is needed [34][35][60,61].
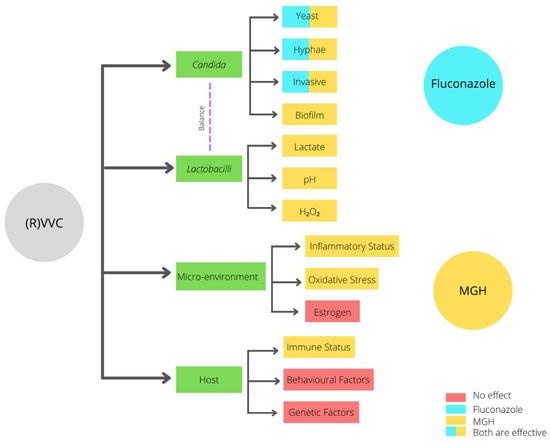
| Characteristic | Fluconazole | MGH |
|---|---|---|
| Candida albicans | + | + |
| (Increased raise in VVC caused by) NAC species | − | + |
| Biofilms | − | + |
| Increased resistance | − | + |
| Microenvironment/vaginal mucosal response | − | + |
| Lactobacilli | − | +− |
| pH | − | + |
| Osmotic effect | − | + |
| Antimicrobial | + | + |
| Anti-inflammatory | − | + |
| Antioxidative | − | + |
Detailed mechanisms of how MGH affects the indicated pathways are described in the original manuscript. Extensive pre-clinical and clinical literature is discussed that supports that MGH is a promising treatment for RVVC. In addition, a new randomized-controlled trial (clinicaltrials.gov NCT04626258) is presented that intents to investigate the mycological and clinical cure and the prophylactic efficacy of the MGH-formulation L-Mesitran Soft in relation to the standard of care (fluconazole). L-Mesitran Soft is CE- and FDA approved and contains 40% MGH and different supplements that consistently have demonstrated to enhance the antimicrobial and wound healing activities.
