
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Shubina | + 2510 word(s) | 2510 | 2021-08-20 09:34:39 | | | |
| 2 | Enzi Gong | Meta information modification | 2510 | 2021-08-23 05:53:50 | | |
Video Upload Options
Polyphenols are the most numerous and widely distributed compounds of plant origin. They are involved in various processes of the growth and development of plants, and their protection against unfavorable environmental factors. They enter the body of humans and animals with plant food. The intake of polyphenols or polyphenol-rich food products might be associated with a lower risk of cardiovascular, neurodegenerative, and other diseases. More than 8000 polyphenols have been identified; of these, more than 4000 compounds belong to the group of flavonoids. In nature, polyphenols occur as monomers, oligomers, and polymers (proanthocyanidins, condensed tannins). There is also evidence indicating that, during the storage and aging of food products and beverages with a high content of flavonoids, the latter react with carbonyl compounds such as acetaldehyde, methylglyoxal, glyoxylic acid, and furfurol, which results in the formation of monomeric, oligomeric, and polymeric adducts.
1. Overview
It is known that flavonoids can react with toxic carbonyl compounds in the process of the storage, aging, and digestion of flavonoid-rich foods and beverages. However, the effect of these reactions on the antioxidant properties of the polyphenolic fraction and the properties of the resulting products remain poorly studied. The aim of the present work was to study the antioxidant activity of quercetin, taxifolin, catechin, eriodictyol, hesperetin, naringenin and a product of the condensation of taxifolin with glyoxylic acid, as well as to reveal the structure–activity relationship of these polyphenols. It was found that flavonoids containing the catechol moiety exhibited higher antioxidant activity than hesperetin and naringenin. The product showed the highest hydrogen peroxide scavenging activity, a lower metal-reducing and a higher iron-binding ability than catechol-containing flavonoids, and a lipid peroxidation inhibitory activity comparable with that of taxifolin. Thus, the condensation of flavonoids with toxic carbonyl compounds might lead to the formation of products exhibiting high antioxidant activity. Meanwhile, the conditions under which parent flavonoids and their products exhibit the maximal antioxidant activity may differ. The data suggest that the antioxidant profile of the polyphenolic fraction and bioavailability of polyphenols, carbonyl compounds, and metal ions may change when these reactions occur.
2. Polyphenols
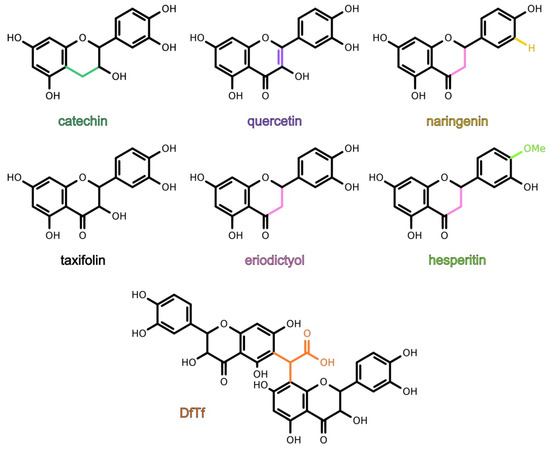
3. Conclusions
References
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246.
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; p. 1237. ISBN 978-0-8493-2021-7.
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30.
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118.
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. ISBN 978-0-12-822919-4.
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between habitual polyphenol intake and incidence of cardiovascular events in the predimed study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647.
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594.
- Johanna Rienks; Janett Barbaresko; Ute nöthlings association of polyphenol biomarkers with cardiovascular disease and mortality risk: A systematic review and meta-analysis of observational studies. Nutrients 2017, 9, 415.
- Lindsay, J. Risk factors for alzheimer’s disease: A prospective analysis from the Canadian study of health and aging. Am. J. Epidemiol. 2002, 156, 445–453.
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143.
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371.
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.-F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363.
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131.
- Goetz, M.E.; Judd, S.E.; Hartman, T.J.; McClellan, W.; Anderson, A.; Vaccarino, V. Flavanone intake is inversely associated with risk of incident ischemic stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. J. Nutr. 2016, 146, 2233–2243.
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem. 2000, 48, 5946–5954.
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Role of aldehydic derivatives in the condensation of phenolic compounds with emphasis on the sensorial properties of fruit-derived foods. J. Agric. Food Chem. 2002, 50, 5571–5585.
- Lo, C.-Y.; Li, S.; Tan, D.; Pan, M.-H.; Sang, S.; Ho, C.-T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128.
- Totlani, V.M.; Peterson, D.G. Epicatechin carbonyl-trapping reactions in aqueous maillard systems: Identification and structural elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318.
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of quercetin and its methylglyoxal adducts on the formation of α-dicarbonyl compounds in a lysine/glucose model system. J. Agric. Food Chem. 2017, 65, 2233–2239.
- Delgado, R.M.; Hidalgo, F.J.; Zamora, R. Antagonism between lipid-derived reactive carbonyls and phenolic compounds in the strecker degradation of amino acids. Food Chem. 2016, 194, 1143–1148.
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Protective effect of phenolic compounds on carbonyl-amine reactions produced by lipid-derived reactive carbonyls. Food Chem. 2017, 229, 388–395.
- Zamora, R.; Aguilar, I.; Hidalgo, F.J. Epoxyalkenal-trapping ability of phenolic compounds. Food Chem. 2017, 237, 444–452.
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49.
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007.
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A Novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46.
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796.
- Sirota, R.; Gorelik, S.; Harris, R.; Kohen, R.; Kanner, J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol. Nutr. Food Res. 2013, 57, 916–919.
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The postprandial oxidative stress index (posi) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936.
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional chinese medicinal plants. Life Sci. 2006, 78, 2872–2888.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956.
- Shubina, V.S.; Shatalin, Y.V. Antioxidant and iron-chelating properties of taxifolin and its condensation product with glyoxylic acid. J. Food Sci. Technol. 2017, 54, 1467–1475.
- Latos-Brozio, M.; Masek, A. Structure-activity relationships analysis of monomeric and polymeric polyphenols (quercetin, rutin and catechin) obtained by various polymerization methods. Chem. Biodivers. 2019, 16.
- Hashemi Gahruie, H.; Niakousari, M. Antioxidant, antimicrobial, cell viability and enzymatic inhibitory of antioxidant polymers as biological macromolecules. Int. J. Biol. Macromol. 2017, 104, 606–617.
- Chung, J.E.; Kurisawa, M.; Kim, Y.-J.; Uyama, H.; Kobayashi, S. Amplification of antioxidant activity of catechin by polycondensation with acetaldehyde. Biomacromolecules 2004, 5, 113–118.
- Kim, Y.-J.; Chung, J.E.; Kurisawa, M.; Uyama, H.; Kobayashi, S. Superoxide anion scavenging and xanthine oxidase inhibition of (+)-catechin-aldehyde polycondensates. amplification of the antioxidant property of (+)-catechin by polycondensation with aldehydes. Biomacromolecules 2004, 5, 547–552.
- Kim, Y.-J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261.
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399.
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Thermally stable and antimicrobial active poly(catechin) obtained by reaction with a cross-linking agent. Biomolecules 2020, 11, 50.
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Novel polymeric biomaterial based on naringenin. Materials 2021, 14, 2142.
- Falleh, H.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Abdelly, C.; Magné, C. Phenolic nature, occurrence and polymerization degree as marker of environmental adaptation in the edible halophyte mesembryanthemum edule. S. Afr. J. Bot. 2012, 79, 117–124.
- Jerez, M.; Touriño, S.; Sineiro, J.; Torres, J.L.; Núñez, M.J. Procyanidins from pine bark: Relationships between structure, composition and antiradical activity. Food Chem. 2007, 104, 518–527.
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532.
- Zhou, H.-C.; Tam, N.F.; Lin, Y.-M.; Ding, Z.-H.; Chai, W.-M.; Wei, S.-D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant ceriops tagal. PLoS ONE 2014, 9, e107606.
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 3′,4′-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(ii) ions: A spectroscopic, absorption titration and dna damage study. Molecules 2019, 24, 4335.
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. prooxidant properties of the flavonoid, kaempferol, in the presence of cu(ii) ions: A ros-scavenging activity, fenton reaction and dna damage study. Int. J. Mol. Sci. 2021, 22, 1619.
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208.
- Shubina, V.S.; Shatalina, Y.V. Absorption spectroscopy study of acid-base and metal-binding properties of flavanones. J. Appl. Spectrosc. 2013, 80, 761–766.
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Effect of complex formation by taxifolin and naringenin with cu(i) ions on the distribution of the components of complexes in the octanol–water system. Russ. J. Bioorganic Chem. 2017, 43, 463–470.
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701.
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111.
- Guo, M.; Perez, C.; Wei, Y.; Rapoza, E.; Su, G.; Bou-Abdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007, 4951.
- Gibbs, C.R. Characterization and application of ferrozine iron reagent as a ferrous iron indicator. Anal. Chem. 1976, 48, 1197–1201.
- Xiao, Z.; Brose, J.; Schimo, S.; Ackland, S.M.; La Fontaine, S.; Wedd, A.G. Unification of the copper(i) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins. J. Biol. Chem. 2011, 286, 11047–11055.
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J. Agric. Food Chem. 2010, 58, 7423–7431.
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332.
- Catapano, M.; Tvrdý, V.; Karlíčková, J.; Migkos, T.; Valentová, K.; Křen, V.; Mladěnka, P. The stoichiometry of isoquercitrin complex with iron or copper is highly dependent on experimental conditions. Nutrients 2017, 9, 1193.
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin b-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937.
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from fenton reaction by complexing ferrous ions1this study is dedicated to the memory of botany professor Luiz, F.G. Labouriau (1921–1996).1. Biochim. Biophys. Acta BBA Gen. Subj. 1999, 1472, 142–152.
- Macáková, K.; Mladěnka, P.; Filipský, T.; Říha, M.; Jahodář, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592.
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta BBA Gen. Subj. 2005, 1721, 174–184.
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402.
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodář, L.; Hrdina, R.; et al. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638.
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486.
- Chen, X.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. J. Am. Oil Chem. Soc. 1998, 75, 1717.
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103.
- Yun, B.-S.; Lee, I.-K.; Kim, J.-P.; Chung, S.-H.; Shim, G.-S.; Yoo, I.-D. Lipid peroxidation inhibitory activity of some constituents isolated from the stem bark ofeucalyptus globulus. Arch. Pharm. Res. 2000, 23, 147–150.
- Brown, J.E.; Kelly, M.F. Inhibition of lipid peroxidation by anthocyanins, anthocyanidins and their phenolic degradation products. Eur. J. Lipid Sci. Technol. 2007, 109, 66–71.
- Foti, M.; Piattelli, M.; Baratta, M.T.; Ruberto, G. Flavonoids, coumarins, and cinnamic acids as antioxidants in a micellar system. structure−activity relationship †. J. Agric. Food Chem. 1996, 44, 497–501.




