Polyphenols are the most numerous and widely distributed compounds of plant origin. They are involved in various processes of the growth and development of plants, and their protection against unfavorable environmental factors. They enter the body of humans and animals with plant food. The intake of polyphenols or polyphenol-rich food products might be associated with a lower risk of cardiovascular, neurodegenerative, and other diseases. More than 8000 polyphenols have been identified; of these, more than 4000 compounds belong to the group of flavonoids. In nature, polyphenols occur as monomers, oligomers, and polymers (proanthocyanidins, condensed tannins). There is also evidence indicating that, during the storage and aging of food products and beverages with a high content of flavonoids, the latter react with carbonyl compounds such as acetaldehyde, methylglyoxal, glyoxylic acid, and furfurol, which results in the formation of monomeric, oligomeric, and polymeric adducts.
1. Overview
It is known that flavonoids can react with toxic carbonyl compounds in the process of the storage, aging, and digestion of flavonoid-rich foods and beverages. However, the effect of these reactions on the antioxidant properties of the polyphenolic fraction and the properties of the resulting products remain poorly studied. The aim of the present work was to study the antioxidant activity of quercetin, taxifolin, catechin, eriodictyol, hesperetin, naringenin and a product of the condensation of taxifolin with glyoxylic acid, as well as to reveal the structure–activity relationship of these polyphenols. It was found that flavonoids containing the catechol moiety exhibited higher antioxidant activity than hesperetin and naringenin. The product showed the highest hydrogen peroxide scavenging activity, a lower metal-reducing and a higher iron-binding ability than catechol-containing flavonoids, and a lipid peroxidation inhibitory activity comparable with that of taxifolin. Thus, the condensation of flavonoids with toxic carbonyl compounds might lead to the formation of products exhibiting high antioxidant activity. Meanwhile, the conditions under which parent flavonoids and their products exhibit the maximal antioxidant activity may differ. The data suggest that the antioxidant profile of the polyphenolic fraction and bioavailability of polyphenols, carbonyl compounds, and metal ions may change when these reactions occur.
2. Polyphenols
Polyphenols are the most numerous and widely distributed compounds of plant origin
[1]. They are involved in various processes of the growth and development of plants
[2], and their protection against unfavorable environmental factors
[3][4][5][3,4,5]. They enter the body of humans and animals with plant food. The intake of polyphenols or polyphenol-rich food products might be associated with a lower risk of cardiovascular
[6][7][8][6,7,8], neurodegenerative
[9][10][11][12][9,10,11,12], and other diseases
[6][13][14][6,13,14]. More than 8000 polyphenols have been identified; of these, more than 4000 compounds belong to the group of flavonoids
[1]. In nature, polyphenols occur as monomers, oligomers, and polymers (proanthocyanidins, condensed tannins). There is also evidence indicating that, during the storage and aging of food products and beverages with a high content of flavonoids, the latter react with carbonyl compounds such as acetaldehyde, methylglyoxal, glyoxylic acid, and furfurol, which results in the formation of monomeric, oligomeric, and polymeric adducts
[15][16][17][18][19][20][21][22][15,16,17,18,19,20,21,22].
When entering the human gastrointestinal tract (GIT), polyphenols either remain in the native form or undergo various chemical transformations
[23]. Both parent and modified polyphenols are capable of producing local effects. In addition, when entering the circulation, they may induce systemic effects
[23]. In the first case, the effect of polyphenols can be appreciable because their concentration in the GIT can reach rather high values
[23][24][25][23,24,25]. In particular, they can cause antioxidant effects by interacting with reactive oxygen species (ROS) and ions of metals of variable valency or react with toxic compounds formed during the preparation and digestion of food. This is evidenced by the literature data indicating that the consumption of some food products containing partially oxidized lipids increases the level of toxic hydroperoxides and malonic aldehyde in the stomach and, as a result of their absorption, in the blood plasma. The combined consumption of these food products with polyphenolic compounds substantially reduces or completely prevents the accumulation of these toxic compounds
[26][27][28][29][30][26,27,28,29,30], producing a protective effect on the body. However, the properties of the resulting compounds as well as the effect of the condensation of polyphenols with carbonyl compounds on the antioxidant properties of the polyphenolic fraction remain poorly understood.
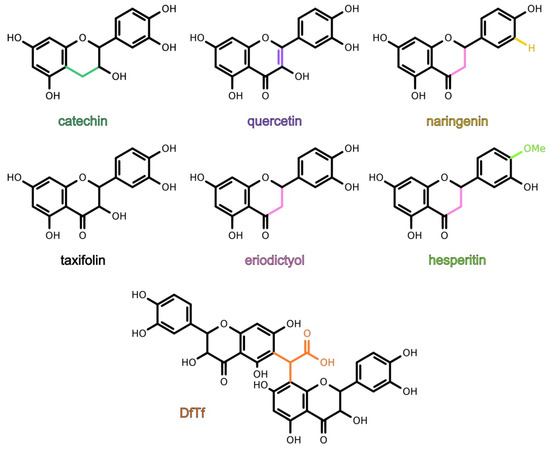
The goal of the present work was to study the antioxidant activity of six structurally similar flavonoids (quercetin, taxifolin, catechin, eriodictyol, hesperetin, naringenin) and a product of the condensation of taxifolin with glyoxylic acid (Figure 1), as well as to reveal the structure–activity relationship of these polyphenols. For this purpose, the ability of the polyphenols to scavenge hydrogen peroxide and inhibit iron-induced lipid peroxidation was examined. In addition, the ability of the polyphenols to reduce iron and cooper ions was estimated.
Figure 1. Structures of polyphenols being tested. The product of the condensation of taxifolin with glyoxylic acid is designated as DfTf.
3. Conclusions
Compounds containing hydroxyl groups in the B ring and being in the
ortho-position relative to each other (the catechol fragment) have the most effective antioxidant activity (DfTf, eriodictyol, quercetin, taxifolin, catechin). The methylation of the hydroxyl group at the 4′-position (hesperetin) or the absence of the hydroxyl group at the 3′-position of the B ring (naringenin) leads to a significant reduction in activity. The presence of the 2,3-double bond, or the carbonyl group at the C4 atom, or the aliphatic hydroxyl group at the 3-position of the C ring affects the antioxidant properties of these compounds substantially less. These results agree well with the literature data indicating that quercetin exhibits high scavenging activity toward radicals generated in the aqueous phase (ABTS and DPPH radicals). Catechin and taxifolin show a lower radical scavenging activity than quercetin, whereas hesperetin and naringenin possess a lower antiradical activity than flavonoids mentioned above
[31][32][35,36]. In spite of the fact that the order of efficacy of flavonoids as radical scavengers can change depending on experimental conditions and methods used by the authors, the most important structural feature of high antiradical activity for these compounds remains the same: the presence of the
ortho-dihydroxy group in the B-ring
[31][32][35,36]. DfTf is a dimer consisting of two taxifolin units, which are linked via the carboxymethine bridge at the C-6 and C-8 positions of the A ring
[33][31]. Consequently, DfTf contains twice as many hydroxyl groups in the structure as the parent flavonoid and, as indicated above, shows the highest hydrogen peroxide scavenging activity. Based on these data and on our previous results, it can be concluded that the effectiveness of DfTf against ROS present in the aqueous phase is higher than that of the parent flavonoid taxifolin
[33][31] and its structural analogs (quercetin, eriodictyol, catechin, hesperetin, and naringenin).
Although the properties of the products of condensation and polymerization of flavonoids remain poorly studied
[34][35][37,38], there is evidence that these compounds exhibit higher antioxidant, antimicrobal, and enzyme inhibitory activities than parent flavonoids
[35][36][37][38][39][40][41][38,39,40,41,42,43,44]. In particular, the polycondensates of catechin-aldehyde and poly(rutin) show higher superoxide-anion scavenging activity and inhibit the oxidation of human low-density lipoprotein to a greater extent than the monomeric form of flavonoids
[36][38][39][39,41,42]. Poly(naringenin) has a greater activity in reducing ABTS and DPPH radicals than naringenin
[41][44]. Proanthocyanidins found in various plants exhibit potent antioxidant activity, which positively correlates with the degree of their polymerization
[42][43][44][45][45,46,47,48]. The products of reactions between quercetin and methylglyoxal (mono- and di-MGO quercetin adducts) retain the ability to scavenge DPPH radical and carbonyl compounds
[19]. Thus, the condensation of flavonoids with toxic lipid peroxidation products leads not only to the utilization of the latter, but also to the formation of compounds exhibiting high antioxidant activity.
The antioxidant activity of polyphenols in turn determines their capacity to reduce transition metal ions, which can participate in the Fenton reaction in the presence of hydrogen peroxide, resulting in the initiation and branching of radical chains
[46][47][49,50]. It is worth noting that, in systems containing polyphenol and transition metal ions, such as Fe (III) and Cu (II), several processes affecting each other may take place simultaneously. In particular, it is known that polyphenols bind transition metal ions, forming complexes with different stoichiometry, a process which highly depends on experimental conditions
[48][49][50][51][52][32,51,52,53,54]. The redox process leads to changes in the composition and the ratio of the components present in the system. As a result, various complexes are composed, among other things, of oxidized forms of flavonoids and reduced metal ions form
[52][53][54,55]. On the other hand, complex formation leads to changes in the redox potential of the system and a shift of the equilibrium of the redox reaction. Reagents used for the estimation of the metal-reducing activity of compounds, such as ferrozine and BCDS, firmly bind reduced metal ions (the constant of binding of ferrozine to Fe
2+ is logβ = 15.5
[54][56] and that of BCDS to Cu
+ is logβ = 19.8
[55][57]), minimizing the effects of processes proceeding simultaneously on the result of the measurement. Nevertheless, if a polyphenol is capable of firmly binding metal ions, it may compete with the reagent (ferrozine, BCDS) for the binding of the corresponding metal ions
[51][53][56][57][58][59][60][53,55,58,59,60,61,62]. In these cases, the concentration of ions that was reduced in the presence of the polyphenol may also be considered as a measure of its redox activity in the reaction mixture and can be used to compare the metal-reducing activity of this polyphenol and other compounds. In turn, the model system containing reagents competing with polyphenols for metal ions offers an additional tool for studying polyphenol–metal ion interaction. The data being obtained by means of these systems form the basis necessary for a better understanding of the mechanisms underlying the pro- and antioxidant effects of polyphenols as well as a switch from antioxidant to prooxidant properties of these compounds
[46][47][49,50].
An examination of the metal-reducing activity of polyphenols showed that copper (II) and iron (III) ions are reduced most effectively in the presence of four compounds containing a catechol fragment: catechin, quercetin, eriodictyol, and taxifolin. In the literature, there is evidence indicating a high metal-reducing activity of catechol-containing compounds (quercetin, taxifolin, catechin)
[48][61][62][32,63,64]. However, the order of metal-reducing efficacy of flavonoids may change depending on pH, the flavonoid/metal ion molar ratio, and the time interval
[61][62][63][63,64,65]. Most likely, in the first two cases, the changes in the redox potentials of the systems play a key role, while in the last case the decisive factor is the kinetics of the redox reactions
[62][64]. In general, the results obtained here and the available literature data demonstrate high metal-reducing activity of polyphenols containing the catechol group
[48][61][62][32,63,64]. DfTf contains two catechol moieties in the structure. Nevertheless, the metal-reducing activity of this compound is lower than that of catechol-containing flavonoids, suggesting that this polyphenol is less likely to act as a prooxidant. The metal-reducing capacity of poly(catechin)s condensed through acetaldehyde was also lower than that of the parent flavonoid and decreased as their molecular weight increased
[36][39]. The copper-reducing activity of poly(naringenin) was lower than that of naringenin
[41][44]. On the other hand, the metal-reducing power of proanthocyanidins increased with increasing size of polyphenols
[56][58].
On the whole, our results support the data indicating that polyphenols (including DfTf) exhibit higher reducing capacity toward copper ions than toward iron ions
[48][32]. It is significant that the concentration of iron (II) ions in the presence of DfTf depends only insignificantly on its concentration in the system, which we have shown earlier at pH 5.4
[33][31]. This effect is not revealed in the presence of other catechol-containing compounds studied (taxifolin, eriodictyol, catechin, and quercetin) and may be associated with the metal-binding capacity of DfTf and its oxidized forms. This assumption is supported by the fact that ferrozine and DfTf compete with each other for the binding of Fe (II) ions; in this case, DfTf rather firmly binds these ions. To date there are no studies devoted to the evaluation of the stability of complexes formed between the conjugates of flavonoids with carbonyl compounds and transition metal ions. However, in the literature there is evidence indicating that some polyphenols compete for binding of the corresponding ions with well-known chelators, including ferrozine. These are baicalein (acidic and neutral pH)
[51][57][53,59], quercetin (neutral pH)
[51][53][58][53,55,60], isorhamnetin and tamarixetin (neutral pH)
[59][61], isoquercetin (slightly acidic and neutral pH)
[58][60], proanthocyanidins
[56][58], and tannic acid
[60][62]. Therefore, both monomeric and polymeric polyphenols are capable of firmly binding transition metal ions. Nevertheless, the data available in the literature do not enable one to directly estimate the impact of condensation reactions on the metal-binding capacity of the polyphenolic fraction. Here, we demonstrate for the first time that the interaction of taxifolin with glyoxylic acid leads to the formation of a product (DfTf) exhibiting higher metal-binding activity in comparison not only to the parent flavonoid but also to its structural analog quercetin, which possesses higher metal-binding capacity than taxifolin
[51][53]. Taking into account the structural features of polyphenols and the data presented in the literature, several iron binding sites of DfTf can be proposed: (1) the 4-carbonyl group in the C ring and the 5-hydroxyl group of the A ring; (2) the 4-carbonyl group in the C ring and the 3-hydroxyl group of the C ring; and (3) the 3′- and 4′-hydroxy groups of the B ring
[46][49][51][64][49,51,53,66]. Our previous results have shown that naringenin lacking the 3-hydroxyl group in the C ring and 3′-hydroxy groups in the B ring
[49][50][51,52] are able to form complexes with iron (II) and copper (I) ions, suggesting that the coordination of metal ions involves the carbonyl and 3-hydroxyl groups. It may be assumed that the coordination of metal ions in DfTf-iron complexes occurs at the same positions. Taxifolin also forms complexes with iron (II) and copper (I) ions
[49][50][51,52]. With due regard for the stoichiometry of the complexes, it may be concluded that, in contrast to naringenin, one molecule of taxifolin is capable of binding two metal ions
[49][50][51,52], indicating that taxifolin has two coordination sites. Presumably, DfTf has similar coordination sites. In favor of this suggestion are our previous data indicating that DfTf is able to bind up to four iron (II) ions
[33][31]. Supposing that one metal ion is coordinated at the carbonyl group in the fourth position of the C ring and at the 5-OH-group, it is more likely that the other ion is coordinated at the 3′- and 4′-hydroxy groups of the B ring. Nevertheless, the involvement of the 3-hydroxyl and 4-oxo groups in the complex formation by the polyphenols cannot be excluded.
A study of LPO in the presence of polyphenols and iron (II) ions showed that quercetin, catechin, eriodictyol, and taxifolin (compounds containing a catechol fragment) are effective inhibitors of LPO and exert similar inhibitory effects. Other studies on the effects of flavonoids on the lipid peroxidation demonstrated a different order of the effectiveness of these compounds, which depends on the system used
[65][66][67,68]. Thus, Shahidi and coworkers have shown that quercetin and taxifolin exhibit similar antioxidant activity in a lipid system
[67][69], while in another study quercetin inhibited the lipid peroxidation to a lesser extent than taxifolin
[68][70]. Quercetin better protects lipids from oxidation induced by ultraviolet radiation or Fe (II) than catechin
[66][68]. In addition, quercetin prevents the autooxidation of rat cerebral membranes better than hesperetin and naringenin
[65][67]. On the other hand, hesperetin prevents linoleate peroxidation induced by Fe (II) better than quercetin
[65][67]. On the whole, the data indicate that compounds containing the
ortho-dihydroxy group exhibit a high lipid peroxidation inhibitory activity
[32][69][70][36,71,72].