
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maurizio Francesco Brivio | + 983 word(s) | 983 | 2020-07-14 04:54:00 | | | |
| 2 | Nora Tang | Meta information modification | 983 | 2020-11-05 13:30:29 | | | | |
| 3 | Nora Tang | + 2 word(s) | 985 | 2020-11-09 07:57:40 | | |
Video Upload Options
Parasitoid wasps inject eggs into the host insect along with several factors that modulate the immune response, in addition these molecular structures and compounds, present at the surface of the gamete, contribute to the evasive and depressive strategies of the parasitoid by facilitating the development of eggs and larvae within the host body.
1. instructions
Entomopathogen wasps are a large group in the Hymenopteran superfamily and are used as effective bioinsecticides against different insect orders, such as Lepidoptera, Coleoptera, Diptera, and Hemiptera. Wasps lay their eggs in the body cavity of their host, where their progeny feed, and permissive hosts die because of wasp development. Many wasp species successfully parasitize one or a limited permissive host species [1][2][3][4][5].
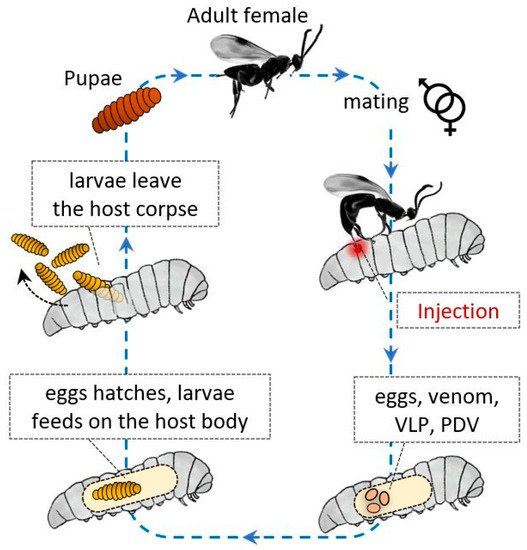
These Hymenoptera have evolved a variety of strategies to avoid host immune responses, and as described for other parasites and microorganisms, these strategies can be divided into passive and active mechanisms. In general, factors of maternal and embryonic origin protect their progeny from the host humoral and cellular immune system responses (Figure 1).
2. Development
After injection, the wasp eggs and developing larvae are supported by various factors, and protective active strategies are carried out by various components, such as polydnaviruses (PDVs) and glycoproteins similar to PDVs, termed virus-like particles (VLPs) [6][7][8][9]. The eggs are injected together with calix fluid, which contains serine protease inhibitors that can inactivate the cellular response of hemocytes [10] and the proPO system [11]; besides, venom components can be coinjected, activating intracellular caspases and inducing apoptosis in the plasmatocytes and lamellocytes of Drosophila melanogaster [12]. All these components can avoid and deactivate the recognition processes, neutralizing the host immune response.
Concerning passive evasion strategies, ichneumonid wasps can implement egg and larval surface protection by means of covering factors [13][14]; these factors do not appear to affect cellular responses, as the host is able to react to wounds with encapsulation processes; in the absence of these protective compounds, wasp eggs are encapsulated, probably due to reactivity against some chorion proteins. The authors demonstrated that the egg surface layers of Cardiochiles nigriceps protect the egg, avoiding recognition by hemocytes; the data suggested that the glycoprotein fibrous layer present on mature eggs of the wasp helps to evade encapsulation by Heliotis virescens hemocytes.
VLPs produced in the calyx of Venturia canescens play a main role in immunoevasion by the wasp, as they cover the egg with a coat immune compliant with the host [7]. VLPs can be considered factors involved in passive evasion [15], even though further studies have identified several VLPs that appear to modulate the host immune physiology [16][17][18].
A glycoprotein called hemomucin plays a central role in passive evasion [19][20]. Hemomucin is an O-glycosylated protein identified on the surface of eggs [20]; it shows specific affinity for hemolymph lipophorin and can form complexes with the lipoprotein that covers the egg; the complexes formed between surface mucin and lipophorin seem to avoid hemocyte recognition, as host cells are unable to adhere to surfaces coated with these molecular complexes [21][22]. Hu et al. [20] demonstrated that O-glycosidase treatment or antibody binding of hemomucin resulted in the loss of protection and encapsulation of wasp eggs.
As previously suggested, wasp eggs and larvae are often covered by surface factors that are not recognized as foreign; consequently, hemocytes do not adhere [23][24] (Figure 2).
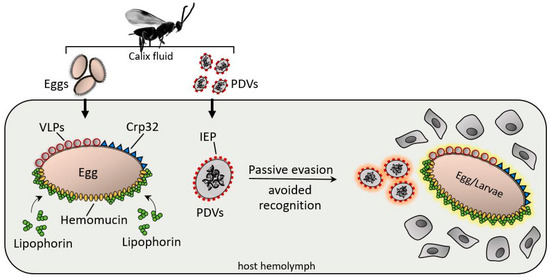
Figure 2. After injection, various surface factors protect the wasp eggs and PDV from the host immune system. Virus IEP (immunoevasive proteins), a thin-layer component covering PDVs; hemomucin, an O-glycosylated transmembrane protein with affinity for host hemolymph proteins (e.g., lipophorins); Crp32, an ovarian component; and VLPs are responsible for immunoevasion strategies, protecting the PDV eggs, and larvae from hemocyte-mediated encapsulation processes.
Cotesia rubecula ovarian proteins have been identified as being clearly involved in passive evasion mechanisms against Pieris rapae immune recognition; in particular, a 32-kDa protein from the ovarian calix shows protective properties for eggs and PDVs [24][25]. Assays have demonstrated that Sephadex beads coated with a recombinant egg surface protein (Crp32) were not encapsulated when injected into hosts, whereas uncoated beads were rapidly encapsulated [26].
Passive evasion mechanisms also involve PDV surfaces; indeed Tanaka et al. [27] identified a protein (IEP) with immunoevasive properties. IEP coats the PDV particles of C. kariyai (CkPDV), protecting the virus from encapsulation by the hemocytes of Mythimna separata; the immunoevasive protein is expressed in both the venom and oviducts, suggesting a relationship in the evasion strategy between wasp tissues [28].
Host immunodepression is mainly carried out by compounds present in the venom, a complex mixture of protein and nonprotein molecules injected into the host during oviposition by the female wasp. Studies on venom composition have led to the identification and functional characterization of several compounds involved in the immune modulation of the host. Even if most of them have not been functionally characterized, it is supposed that they could be involved in venom homeostasis, transient paralysis, cytotoxicity, and hemocyte inactivation. A well-studied example is Pimpla hypochondriaca venom, which consists of several enzymes, protease inhibitors, neurotoxin-like, and inhibitors of hemocyte aggregation. Venom compounds from Leptopilina boulardi, such as the proteins RhoGAP and LbSPNy, lead to suppression of Drosophila host cellular encapsulation and melanization, respectively [29]; moreover, calreticulin from C. rubecula venom is responsible for hemocyte inactivation [30]. A further depressive mechanism was described in the eggs of several entomopathogen wasps, which upon hatching, produce and release cells called teratocytes that are responsible for immunodepression. For example, along with other maternal factors, Cotesia plutellae teratocytes play a role in depressing host immunity; these cells synthesize and secrete immunodepressive factors inhibiting the nodulation processes in the lepidopteran P. xylostella [31]. Besides, these cells are also known for their trophic functions and for altering both host development and metamorphosis [32][33].
References
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bioessays 2001, 23, 344–351.
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258.
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu. Rev. Entomol. 2011, 56, 313–335.
- Falabella, P. The mechanism utilized by Toxoneuron nigriceps in inhibiting the host immune system. Invertebr. Surviv. J. 2018, 15, 240–255.
- Yin, C.; Li, M.; Hu, J.; Lang, K.; Chen, Q.; Liu, J.; Guo, D.; He, K.; Dong, Y.; Luo, J.; et al. The genomic features of parasitism, Polyembryony and immune evasion in the endoparasitic wasp Macrocentrus cingulum. BMC Genom. 2018, 19, 420.
- Bedwin, O. An insect glycoprotein: A study of the particles responsible for the resistance of a parasitoid’s egg to the defence reactions of its insect host. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1979, 205, 271–286.
- Feddersen, I.; Sander, K.; Schmidt, O. Virus-like particles with host protein-like antigenic determinants protect an insect parasitoid from encapsulation. EXS 1986, 42, 1278–1281.
- Morales, J.; Chiu, H.; Oo, T.; Plaza, R.; Hoskins, S.; Govind, S. Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J. Insect Physiol. 2005, 51, 181–195.
- Ye, X.Q.; Shi, M.; Huang, J.H.; Chen, X.X. Parasitoid polydnaviruses and immune interaction with secondary hosts. Dev. Comp. Immunol. 2018, 83, 124–129.
- Beck, M.; Theopold, U.; Schmidt, O. Evidence for serine protease inhibitor activity in the ovarian calyx fluid of the endoparasitoid Venturia canescens. J. Insect Physiol. 2000, 46, 1275–1283.
- Zhang, G.; Lu, Z.Q.; Jiang, H.; Asgari, S. Negative regulation of prophenoloxidase (proPO) activation by a clip-domain serine proteinase homolog (SPH) from endoparasitoid venom. Insect Biochem. Mol. Biol. 2004, 34, 477–483.
- Wan, B.; Goguet, E.; Ravallec, M.; Pierre, O.; Lemauf, S.; Volkoff, A.N.; Gatti, J.L.; Poirié, M. Venom Atypical Extracellular Vesicles as Interspecies Vehicles of Virulence Factors Involved in Host Specificity: The Case of a Drosophila Parasitoid Wasp. Front. Immunol. 2019, 10, 1688.
- Rotheram, S. Immune surface of eggs of a parasitic insect. Nature 1967, 214, 700.
- Davies, D.H.; Vinson, S.B. Passive evasion by eggs of braconid parasitoid Cardiochiles nigriceps of encapsulation in vitro by haematocytes of host Heliothis virescens. Possible role for fibrous layer in immunity. J. Insect Physiol. 1986, 32, 1003.
- Reineke, A.; Asgari, S.; Schmidt, O. Evolutionary origin of Venturia canescens virus-like particles. Arch. Insect Biochem. Physiol. 2006, 61, 123–133.
- Hellers, M.; Beck, M.; Theopold, U.; Kamei, M.; Schmidt, O. Multiple alleles encoding a virus-like particle protein in the ichneumonid endoparasitoid Venturia canescens. Insect Mol. Biol. 1996, 5, 239–249.
- Theopold, U.; Krause, E.; Schmidt, O. Cloning of a VLP-protein coding gene from a parasitoid wasp Venturia canescens. Arch. Insect Biochem. Physiol. 1994, 26, 137–145.
- Asgari, S.; Reineke, A.; Beck, M.; Schmidt, O. Isolation and characterization of a neprilysin-like protein from Venturia canescens virus-like particles. Insect Mol. Biol. 2002, 11, 477–485.
- Theopold, U.; Samakovlis, C.; Erdjument-Bromage, H.; Dillon, N.; Axelsson, B.; Schmidt, O.; Tempst, P.; Hultmark, D. Helix pomatia lectin, an inducer of Drosophila immune response, binds to hemomucin, a novel surface mucin. J. Biol. Chem. 1996, 271, 12708–12715.
- Hu, J.; Xu, Q.; Hu, S.; Yu, X.; Liang, Z.; Zhang, W. Hemomucin, an O-glycosylated protein on embryos of the wasp Macrocentrus cingulum that protects it against encapsulation by hemocytes of the host Ostrinia furnacalis. J. Innate Immun. 2014, 6, 663–675.
- Coodin, S.; Caveney, S. Lipophorin inhibits adhesion of cockroach (Periplaneta americana) haemocytes in vitro. J. Insect Physiol. 1992, 38, 853–862.
- Mandato, C.A.; Diehl-Jones, W.L.; Downer, R.G.H. Insect hemocyte adhesion in vitro inhibition by apoliphorin I and an artificial substrate. J. Insect Physiol. 1996, 42, 143–148.
- Davies, D.H.; Burghardt, R.L.; Vinson, S.B. Oogenesis of Cardiochiles nigriceps viereck (Hymenoptera: Braconidae): Histochemistry and development of the chorion with special reference to the fibrous layer. Int. J. Insect Morphol. Embryol. 1986, 15, 363–374.
- Asgari, S.; Schmidt, O. Passive protection of eggs from the parasitoid, Cotesia rubecula, in the host, Pieris rapae. J. Insect Physiol. 1994, 40, 789–795.
- Teng, Z.; Wu, H.; Ye, X.; Xiong, S.; Xu, G.; Wang, F.; Fang, Q.; Ye, G. An Ovarian Protein Involved in Passive Avoidance of an Endoparasitoid to Evade Its Host Immune Response. J. Proteome Res. 2019, 18, 2695–2705.
- Asgari, S.; Theopold, U.; Wellby, C.; Schmidt, O. A protein with protective properties against the cellular defense reactions in insects. Proc. Natl. Acad. Sci. USA 1998, 95, 3690–3695.
- Tanaka, K.; Matsumoto, H.; Hayakawa, Y. Detailed characterization of polydnavirus immunoevasive proteins in an endoparasitoid wasp. Eur. J. Biochem. 2002, 269, 2557–2566.
- Furihata, S.; Tanaka, K.; Ryuda, M.; Ochiai, M.; Matsumoto, H.; Csikos, G.; Hayakawa, Y. Immunoevasive protein (IEP)-containing surface layer covering polydnavirus particles is essential for viral infection. J. Invertebr. Pathol. 2014, 115, 26–32.
- Moreau, S.J.; Asgari, S. Venom Proteins from Parasitoid Wasps and Their Biological Functions. Toxins 2015, 7, 2385–2412.
- Zhang, G.; Schmidt, O.; Asgari, S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 2006, 30, 756–764.
- Andrew, N.; Basio, M.; Kim, Y. Additive effect of teratocyte and calyx fluid from Cotesia plutellae on immunosuppression of Plutella xylostella. Physiol. Entomol. 2006, 31, 341–347.
- Ali, M.R.; Seo, J.; Lee, D.; Kim, Y. Teratocyte-secreting proteins of an endoparasitoid wasp, Cotesia plutellae, prevent host metamorphosis by altering endocrine signals. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 251–262.
- Strand, M.R. Teratocytes and their functions in parasitoids. Curr. Opin. Insect Sci. 2014, 6, 68–73.




