Parasitoid wasps inject eggs into the host insect along with several factors that modulate the immune response, in addition these molecular structures and compounds, present at the surface of the gamete, contribute to the evasive and depressive strategies of the parasitoid by facilitating the development of eggs and larvae within the host body.
- wasp
- parasitoid
- entomopathogen
- insect host
- immunoevasion
- immunodepression
- polyDNAvirus
- host immunity
1. instructions
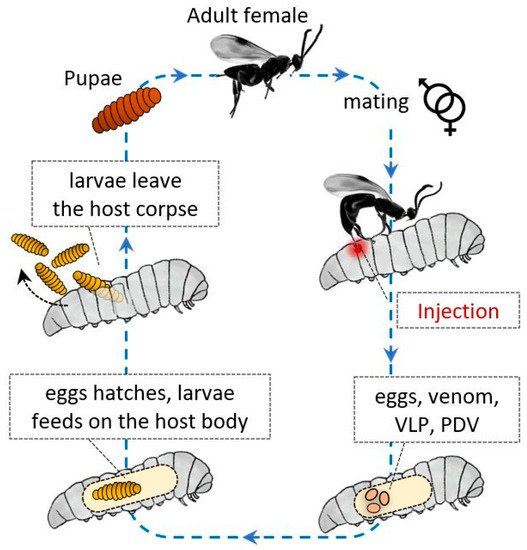
Entomopathogen wasps are a large group in the Hymenopteran superfamily and are used as effective bioinsecticides against different insect orders, such as Lepidoptera, Coleoptera, Diptera, and Hemiptera. Wasps lay their eggs in the body cavity of their host, where their progeny feed, and permissive hosts die because of wasp development. Many wasp species successfully parasitize one or a limited permissive host species [1][2][3][4][5].
These Hymenoptera have evolved a variety of strategies to avoid host immune responses, and as described for other parasites and microorganisms, these strategies can be divided into passive and active mechanisms. In general, factors of maternal and embryonic origin protect their progeny from the host humoral and cellular immune system responses (
Figure 1).
2. Development
After injection, the wasp eggs and developing larvae are supported by various factors, and protective active strategies are carried out by various components, such as polydnaviruses (PDVs) and glycoproteins similar to PDVs, termed virus-like particles (VLPs) [6][7][8][9]. The eggs are injected together with calix fluid, which contains serine protease inhibitors that can inactivate the cellular response of hemocytes [10] and the proPO system [11]; besides, venom components can be coinjected, activating intracellular caspases and inducing apoptosis in the plasmatocytes and lamellocytes of
Drosophila melanogaster [12]. All these components can avoid and deactivate the recognition processes, neutralizing the host immune response.
Concerning passive evasion strategies, ichneumonid wasps can implement egg and larval surface protection by means of covering factors [13][14]; these factors do not appear to affect cellular responses, as the host is able to react to wounds with encapsulation processes; in the absence of these protective compounds, wasp eggs are encapsulated, probably due to reactivity against some chorion proteins. The authors demonstrated that the egg surface layers of
Cardiochiles nigriceps
Heliotis virescens
Venturia canescens play a main role in immunoevasion by the wasp, as they cover the egg with a coat immune compliant with the host [7]. VLPs can be considered factors involved in passive evasion [15], even though further studies have identified several VLPs that appear to modulate the host immune physiology [16][17][18].
A glycoprotein called hemomucin plays a central role in passive evasion [19][20]. Hemomucin is an
O-glycosylated protein identified on the surface of eggs [20]; it shows specific affinity for hemolymph lipophorin and can form complexes with the lipoprotein that covers the egg; the complexes formed between surface mucin and lipophorin seem to avoid hemocyte recognition, as host cells are unable to adhere to surfaces coated with these molecular complexes [21][22]. Hu et al. [20] demonstrated that
O
As previously suggested, wasp eggs and larvae are often covered by surface factors that are not recognized as foreign; consequently, hemocytes do not adhere [23][24] (
Figure 2).
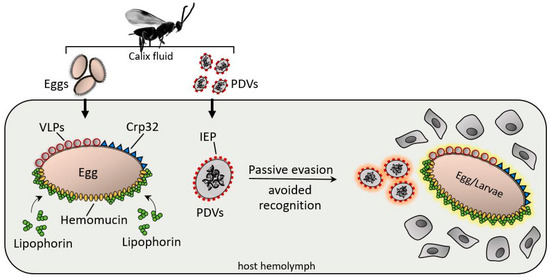
Figure 26.
After injection, various surface factors protect the wasp eggs and PDV from the host immune system. Virus IEP (immunoevasive proteins), a thin-layer component covering PDVs; hemomucin, anO-glycosylated transmembrane protein with affinity for host hemolymph proteins (e.g., lipophorins); Crp32, an ovarian component; and VLPs are responsible for immunoevasion strategies, protecting the PDV eggs, and larvae from hemocyte-mediated encapsulation processes.
-glycosylated transmembrane protein with affinity for host hemolymph proteins (e.g., lipophorins); Crp32, an ovarian component; and VLPs are responsible for immunoevasion strategies, protecting the PDV eggs, and larvae from hemocyte-mediated encapsulation processes.
Cotesia rubecula
Pieris rapae immune recognition; in particular, a 32-kDa protein from the ovarian calix shows protective properties for eggs and PDVs [24][25]. Assays have demonstrated that Sephadex beads coated with a recombinant egg surface protein (Crp32) were not encapsulated when injected into hosts, whereas uncoated beads were rapidly encapsulated [26].
Passive evasion mechanisms also involve PDV surfaces; indeed Tanaka et al. [27] identified a protein (IEP) with immunoevasive properties. IEP coats the PDV particles of
C. kariyai
Mythimna separata; the immunoevasive protein is expressed in both the venom and oviducts, suggesting a relationship in the evasion strategy between wasp tissues [28].
Pimpla hypochondriaca
Leptopilina boulardi
Drosophila host cellular encapsulation and melanization, respectively [29]; moreover, calreticulin from
C. rubecula venom is responsible for hemocyte inactivation [30]. A further depressive mechanism was described in the eggs of several entomopathogen wasps, which upon hatching, produce and release cells called teratocytes that are responsible for immunodepression. For example, along with other maternal factors,
Cotesia plutellae
P. xylostella [31]. Besides, these cells are also known for their trophic functions and for altering both host development and metamorphosis [32][33].
