
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gomaa A. M. Ali | + 2134 word(s) | 2134 | 2021-08-18 16:05:47 | | | |
| 2 | Vicky Zhou | -95 word(s) | 2039 | 2021-08-19 06:42:55 | | | | |
| 3 | Vicky Zhou | Meta information modification | 2039 | 2021-08-20 10:00:00 | | | | |
| 4 | Vicky Zhou | Meta information modification | 2039 | 2021-08-20 10:00:20 | | | | |
| 5 | Vicky Zhou | Meta information modification | 2039 | 2021-08-20 10:01:04 | | |
Video Upload Options
Nanoremediation technologies involve the use of reactive NPs for the conversion and detoxification of contaminants. The main mechanisms for remediation by NPs are catalysis and chemical reduction. In addition, adsorption is another removal mechanism facilitated by the NPs since NPs have high surface-area-to-mass ratios and different distribution of active sites, increasing the adsorption ability.
1. Introduction
2. Relationship between Soil and Groundwater: Contaminants and Remediation
Soil and groundwater are susceptible to pollution by a wide array of pollutants such as petroleum hydrocarbon, chlorinated solvents, and heavy metals. [16]. Selecting a proper remediation technology for a contaminated environment usually depends on contaminant characteristics and contaminated site characteristics such as physical, chemical, and biological properties. All these factors should be considered during the remediation process, design, and implication. Moreover, the time/cost constraints, the regulatory requirements, and the remediation mechanisms should be considered in the selection process.
3. Combined Nanoremediation with Other Remediation Technology
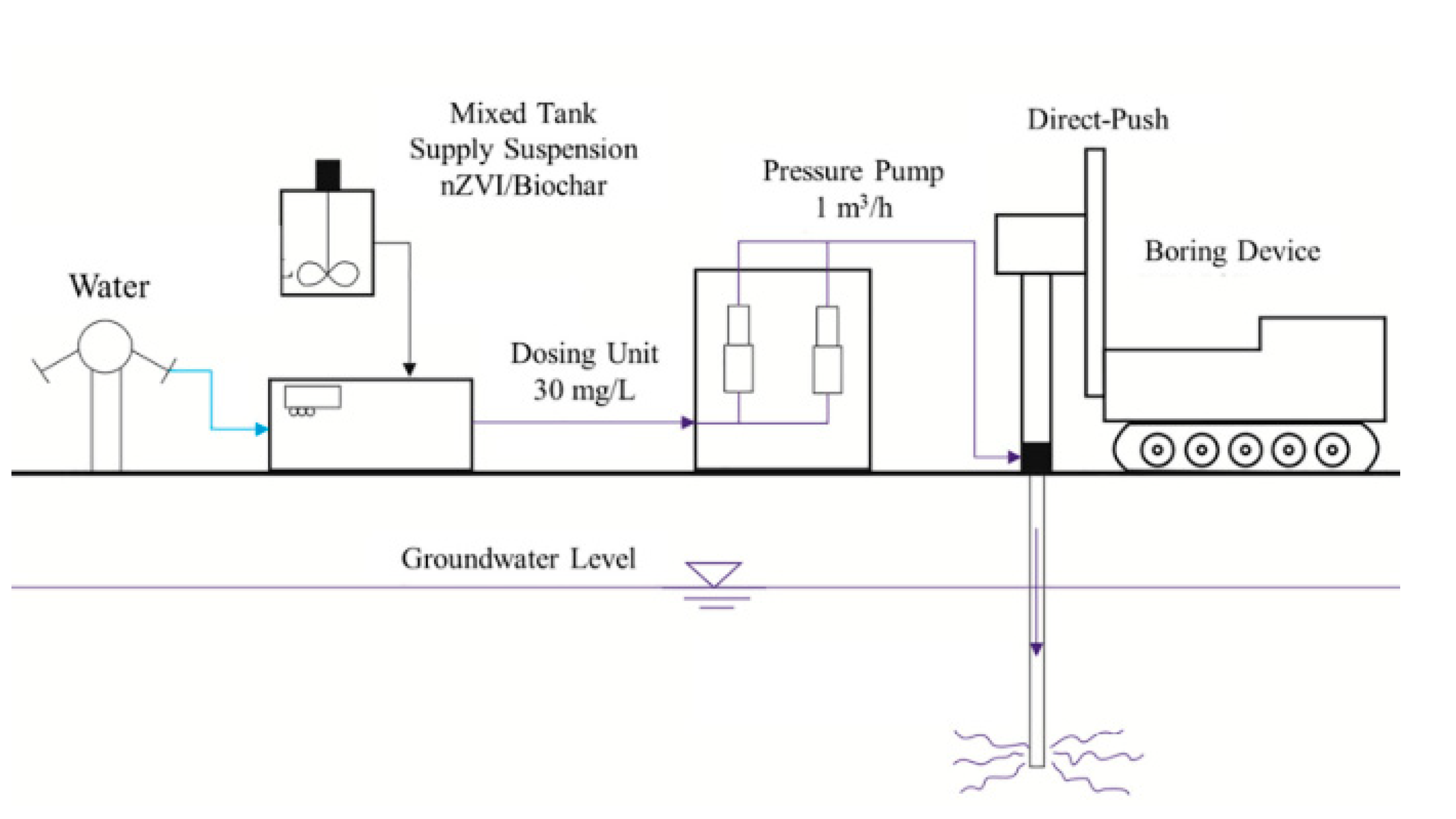
Figure 1. Step of the injection procedure. Reprinted with permission from [29] (2020, Elsevier).
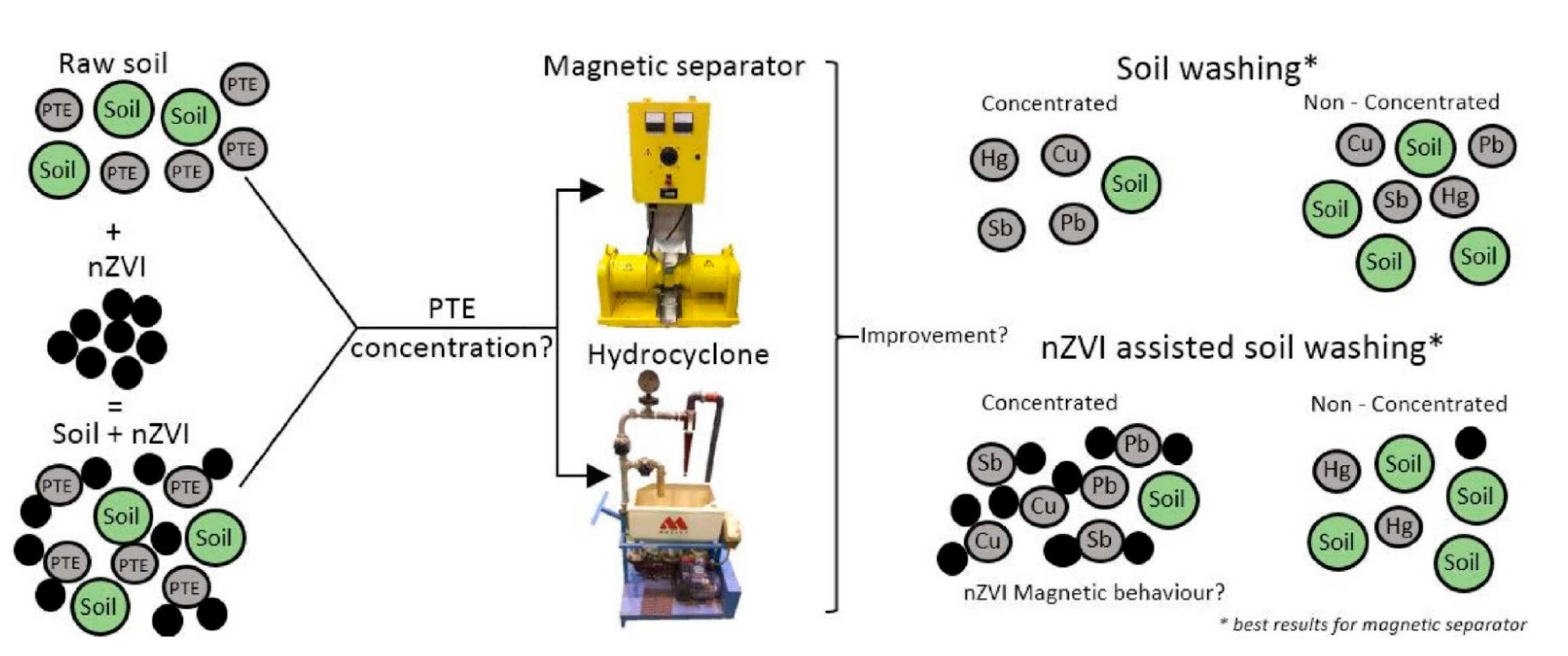
Figure 2. Soil washing assisted nZVI nanoremediation. Reprinted with permission from [32] (2018, Elsevier).
Qu et al. [33] studied the implication of an activated carbon fiber (ACF)-supported nZVI (ACF-nZVI) composite for Cr(VI) remediation from groundwater [33]. In addition, they examined the effect of the operation condition such as nZVI amount on activated carbon fiber, initial Cr(VI) concentration, and pH value on the Cr(VI) removal by conducting a batch experiment. The results indicated that the aggregation of nZVI could be inhabited by ACF, which increases the nZVI reactivity and Cr(VI) removal efficiency. The removal efficiency of Cr(VI) decreased with increasing Cr(VI) initial concentration, whereas, in an acidic environment, complete removal (100%) of Cr(VI) was observed in 1 h reaction time. The proposed removal mechanism consisted of two steps: the first step was the physical adsorption of Cr(VI) on the ACF-nZVI surface area or inner layer, where the second step was a reduction of Cr(VI) to Cr(III) by nZVI [33]. In another study, Huang et al. [34] studied the activation of persulfate (PS) by using a zeolite-supported nZVI composites (PS-Z/nZVI) system and examined its efficiency for TCE degradation. The results indicated that Z/nZVI showed high ability towards PS activation (1.5 mM), and high removal efficiency (98.8%) of TCE was observed at pH 7 within 2 h. Moreover, the PS-Z/nZVI system showed high efficiency in terms of TCE for a wide range of pH (4–7) [34].
4. Conclusions
References
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in Situ Remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1813–1831.
- Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. Regeneration and Reuse of Polymeric Nanocomposites in Wastewater Remediation: The Future of Economic Water Management; Springer: Berlin/Heidelberg, Germany, 2019; Volume 76, pp. 647–681.
- Chaturvedi, S.; Dave, P.N. Water purification using nanotechnology an emerging opportunities. Chem. Methodol. 2019, 3, 115–144.
- Kumar, A.; Joshi, H.; Kumar, A. Remediation of Arsenic by Metal/Metal Oxide Based Nanocomposites/Nanohybrids: Contamination Scenario in Groundwater, Practical Challenges, and Future Perspectives. Sep. Purif. Rev. 2020, 50, 283–314.
- Blundell, S.P.; Owens, G. Evaluation of enhancement techniques for the dechlorination of DDT by nanoscale zero-valent iron. Chemosphere 2021, 264, 128324.
- Chen, J.; Dong, H.; Tian, R.; Li, R.; Xie, Q. Remediation of Trichloroethylene-Contaminated Groundwater by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar: Investigation of Critical Factors. Water Air Soil Pollut. 2020, 231, 432.
- Gil-Díaz, M.; Álvarez, M.A.; Alonso, J.; Lobo, M.C. Effectiveness of nanoscale zero-valent iron for the immobilization of Cu and/or Ni in water and soil samples. Sci. Rep. 2020, 10, 15927.
- Mpouras, T.; Polydera, A.; Dermatas, D.; Verdone, N.; Vilardi, G. Multi wall carbon nanotubes application for treatment of Cr(VI)-contaminated groundwater; Modeling of batch & column experiments. Chemosphere 2021, 269, 128749.
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817.
- Araújo, R.; Castro, A.C.M.; Fiúza, A. The Use of Nanoparticles in Soil and Water Remediation Processes. Mater. Today Proc. 2015, 2, 315–320.
- Mukhopadhyay, R.; Sarkar, B.; Khan, E.; Alessi, D.S.; Biswas, J.K.; Manjaiah, K.M.; Eguchi, M.; Wu, K.C.W.; Yamauchi, Y.; Ok, Y.S. Nanomaterials for sustainable remediation of chemical contaminants in water and soil. Crit. Rev. Environ. Sci. Technol. 2021, 1–50.
- Savolainen, K.; Alenius, H.; Norppa, H.; Pylkkänen, L.; Tuomi, T.; Kasper, G. Risk assessment of engineered nanomaterials and nanotechnologies—A review. Toxicology 2010, 269, 92–104.
- Ganie, A.S.; Bano, S.; Khan, N.; Sultana, S.; Rehman, Z.; Rahman, M.M.; Sabir, S.; Coulon, F.; Khan, M.Z. Nanoremediation technologies for sustainable remediation of contaminated environments: Recent advances and challenges. Chemosphere 2021, 275, 130065.
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21.
- Pak, T.; de Lima Luz, L.F.; Tosco, T.; Costa, G.S.R.; Rosa, P.R.R.; Archilha, N.L. Pore-scale investigation of the use of reactive nanoparticles for in situ remediation of contaminated groundwater source. Proc. Natl. Acad. Sci. USA 2020, 117, 13366–13373.
- Galdames, A.; Mendoza, A.; Orueta, M.; de Soto García, I.S.; Sánchez, M.; Virto, I.; Vilas, J.L. Development of new remediation technologies for contaminated soils based on the application of zero-valent iron nanoparticles and bioremediation with compost. Resour. Effic. Technol. 2017, 3, 166–176.
- Thavamani, P.; Smith, E.; Kavitha, R.; Mathieson, G.; Megharaj, M.; Srivastava, P.; Naidu, R. Risk based land management requires focus beyond the target contaminants—A case study involving weathered hydrocarbon contaminated soils. Environ. Technol. Innov. 2015, 4, 98–109.
- Anyika, C.; Abdul Majid, Z.; Ibrahim, Z.; Zakaria, M.P.; Yahya, A. The impact of biochars on sorption and biodegradation of polycyclic aromatic hydrocarbons in soils—A review. Environ. Sci. Pollut. Res. 2015, 22, 3314–3341.
- Shayegan, H.; Ali, G.A.M.; Safarifard, V. Recent Progress in the Removal of Heavy Metal Ions from Water Using Metal-Organic Frameworks. ChemistrySelect 2020, 5, 124–146.
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J.B.P. Removal of Heavy Metal Water Pollutants (Co2+ and Ni2+) Using Polyacrylamide/Sodium Montmorillonite (PAM/Na-MMT) Nanocomposites. ACS Omega 2019, 4, 10834–10844.
- Cameselle, C.; Chirakkara, R.A.; Reddy, K.R. Electrokinetic-enhanced phytoremediation of soils: Status and opportunities. Chemosphere 2013, 93, 626–636.
- Rodrigo, M.A.; Oturan, N.; Oturan, M.A. Electrochemically Assisted Remediation of Pesticides in Soils and Water: A Review. Chem. Rev. 2014, 114, 8720–8745.
- Chang, K.-S.; Lo, W.-H.; Lin, W.-M.; Wen, J.-X.; Yang, S.-C.; Huang, C.-J.; Hsieh, H.-Y. Microwave-Assisted Thermal Remediation of Diesel Contaminated Soil. Eng. J. 2016, 20, 93–100.
- Camenzuli, D.; Freidman, B.L.; Statham, T.M.; Mumford, K.A.; Gore, D.B. On-site and in situ remediation technologies applicable to metal-contaminated sites in Antarctica and the Arctic: A review. Polar Res. 2013, 32, 21522.
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526.
- Vilardi, G.; Mpouras, T.; Dermatas, D.; Verdone, N.; Polydera, A.; Di Palma, L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere 2018, 201, 716–729.
- Zhang, R.; Zhang, N.; Fang, Z. In situ remediation of hexavalent chromium contaminated soil by CMC-stabilized nanoscale zero-valent iron composited with biochar. Water Sci. Technol. 2018, 77, 1622–1631.
- Zhang, Y.; Yang, J.; Zhong, L.; Liu, L. Effect of multi-wall carbon nanotubes on Cr(VI) reduction by citric acid: Implications for their use in soil remediation. Environ. Sci. Pollut. Res. 2018, 25, 23791–23798.
- Qian, L.; Chen, Y.; Ouyang, D.; Zhang, W.; Han, L.; Yan, J.; Kvapil, P.; Chen, M. Field demonstration of enhanced removal of chlorinated solvents in groundwater using biochar-supported nanoscale zero-valent iron. Sci. Total Environ. 2020, 698, 134215.
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297.
- Czinnerová, M.; Vološčuková, O.; Marková, K.; Ševců, A.; Černík, M.; Nosek, J. Combining nanoscale zero-valent iron with electrokinetic treatment for remediation of chlorinated ethenes and promoting biodegradation: A long-term field study. Water Res. 2020, 175, 115692.
- Boente, C.; Sierra, C.; Martínez-Blanco, D.; Menéndez-Aguado, J.M.; Gallego, J.R. Nanoscale zero-valent iron-assisted soil washing for the removal of potentially toxic elements. J. Hazard. Mater. 2018, 350, 55–65.
- Qu, G.; Kou, L.; Wang, T.; Liang, D.; Hu, S. Evaluation of activated carbon fiber supported nanoscale zero-valent iron for chromium (VI) removal from groundwater in a permeable reactive column. J. Environ. Manag. 2017, 201, 378–387.
- Huang, J.; Yi, S.; Zheng, C.; Lo, I.M.C. Persulfate activation by natural zeolite supported nanoscale zero-valent iron for trichloroethylene degradation in groundwater. Sci. Total Environ. 2019, 684, 351–359.




