
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brandon Nokes | + 1437 word(s) | 1437 | 2021-07-28 13:24:34 | | | |
| 2 | Beatrix Zheng | + 353 word(s) | 1790 | 2021-08-07 16:51:09 | | |
Video Upload Options
Hematopoietic stem cell transplant (HSCT) is a multi-step process with a high risk for complications during marrow ablation, during engraftment, or afterwards. Successful transplantation depends on the selection of the hematopoietic stem cell source, host preparation (conditioning regimen), and modulation of immune cell engraftment to minimize graft-versus-host disease (GVHD).
1. Introduction
Hematopoietic stem cell transplant (HSCT) involves replacing a patient’s bone marrow with hematopoietic stem or progenitor cells from peripheral blood, bone marrow, or umbilical cord from another person or the same individual to restore immune–hematopoietic function after the underlying disease is eliminated [1]. Pulmonary complications post-HSCT affect between 45% and 60% of recipients [2][3] with a mortality rate exceeding 60% in mechanically ventilated patients after autologous HSCT [4]. This review is intended to form a framework for diagnosing and treating non-infectious and infectious pulmonary complications post-HSCT for the general clinician.
2. Hematopoietic Stem Cell Transplant Overview
Hematopoietic stem cell transplant (HSCT) is a multi-step process with a high risk for complications during marrow ablation, during engraftment, or afterwards [5][6][7]. Successful transplantation depends on the selection of the hematopoietic stem cell source, host preparation (conditioning regimen), and modulation of immune cell engraftment to minimize graft-versus-host disease (GVHD) [7].
Advantages of allogeneic transplant include the ability to correct congenital or acquired defects and the immunologic, anti-tumor effects that can occur in response to persistent disease referred to as the graft versus tumor (GVT) effect [8]. Allogeneic transplant requires that the products be HLA-matched, as poorly HLA-matched products can lead to immune dysregulation and increased risk of GVHD. Furthermore, immune recovery is slower, and opportunistic infections are more common.
After selecting the source of stem cells, the host is prepared via high dose myeloablation therapy, reduced intensity myeloablation, or non-myeloablative conditioning [9]. Once a patient undergoes HSCT, engraftment occurs within a conditioning and host-dependent timeframe.
While there are several definitions of successful engraftment, patients typically develop a neutrophil count greater than 500/mm 3, a platelet count greater than 20,000/microliter without any transfusions for one week, and a hematocrit greater than 25% for at least 20 days without any transfusions [10]. For autologous HSCT, white blood cell recovery typically occurs within two weeks while red blood cell and platelet recovery varies from patient to patient [6]. For allogeneic HSCT, peripheral blood granulocyte counts usually show signs of recovery within three weeks while platelet recovery is often delayed, taking on average 5–7 weeks [6]. Neutrophil engraftment is also dependent on the immunosuppressive regimen used, and recovery can average 10–30 days [11].
3. Timeline of Complications Following HSCT
The diagnosis and management of post-HSCT pulmonary manifestations requires a multidisciplinary approach. Diagnosis can be challenging as post-HSCT syndromes often have nonspecific clinical presentations. Utilizing common timelines of illness presentation post-HSCT ( Figure 1 ) in combination with exposures to infectious agents, prior anti-microbial use, and unique radiographic findings aid in the diagnosis. Use of non-invasive tests including upper respiratory cultures, respiratory pathogen PCR, and serologies are useful adjuncts and can take the place of invasive testing to identify infectious pathogens [12]. Timely coordination and open communication between primary service, hematology–oncology, pulmonary, radiology, pathology, and infectious disease specialists is vital to the management of these patients.
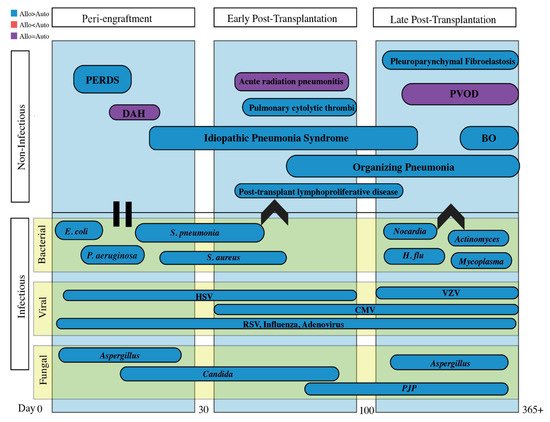
Infectious and non-infectious pulmonary complications are classified by etiology and temporality in reference to HSCT, as it reflects the immunologic state of the patient. The often-cited time course for non-infectious entities consists of the pre/peri-engraftment phase (first 30 days), early post-transplantation phase (30–100 days), and the late post-transplantation phase (after 100 days). Infectious complications will be discussed separately.
4. Infectious Complications
The peri-engraftment phase is hallmarked by mucositis due to conditioning therapy and prolonged neutropenia due to non-functional transplanted marrow [13]. The risk of infection is highest when absolute neutrophil count (ANC) is less than 500 and increases with increasing duration of neutropenia before engraftment [14]. During the early post-transplantation phase, infections are related to the defects in cellular immunity caused by immunosuppressive and conditioning regimens [13]. In the late post-transplantation phase, infection rates decline as immunosuppressant medications are tapered unless needed for cGVHD. Because of differences in immune recovery, autologous HSCT recipients are at higher risk of infectious complications during the peri-engraftment and early post-transplantation phase while allogeneic HSCT recipients are at higher risk of infection throughout the late post-transplantation phase due to cGVHD and prolonged immunosuppressive therapy [15][13].
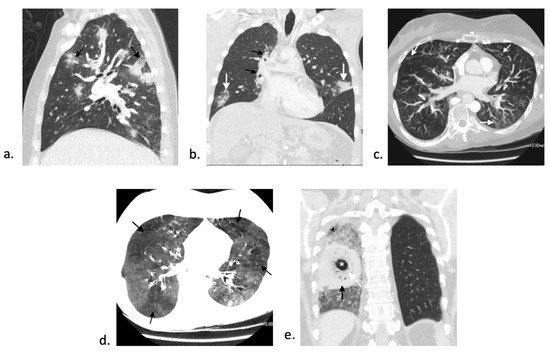
Zygomycosis is the second most common mold infection in HSCT with an increasing incidence over the past few decades [16]. Rare infections with fusarium and scedosporium can occur as well [17]. Invasive fungal infections with mucormycosis generally occurs > 3 months post-transplant and can make up to 8% of fungal infections in this patient population [18]. Although there are various imaging appearances of mucormycosis, one nearly pathognomonic imaging manifestation is the “bird’s nest” sign ( Figure 2 e) [19]. While mucormycosis infections are rare, they are frequently fatal and require early, aggressive management. Treatment involves combination of medical (with amphotericin B for zygomycosis) and surgical therapy [17].
Viral pneumonia from reactivation is common during the peri-engraftment phase and should be closely monitored while patients are immunosuppressed. Viral infection from herpes simplex virus (HSV)-1 and -2 occur in peri-engraftment and early post-transplantation phase, those from cytomegalovirus (CMV) and human herpes virus-6 (HHV-6) are often present in early post-transplantation phase, and infection from varicella zoster virus (VZV) is more common in the late post-transplantation phase [20]. The incidence of reactivation of viruses such as HSV, CMV, and VZV has drastically decreased since prophylaxis became routine.
RSV is one of the more common CARV that is associated with high mortality rate. In a single center retrospective study of 280 allo-HSCT recipients with RSV infection, 29% had progression to lower respiratory tract infection [21]. Older age, smoking history, conditioning with high-dose total body irradiation, and lymphopenia were associated with an increased risk of progression from upper respiratory to lower respiratory tract infection [21][22]. RSV is associated with a wide mortality rate, ranging from 5% to 43%, in HSCT patients [23][21][24][25][26]. Older age, male gender, bone marrow or cord blood as transplant source, corticosteroid use, lower respiratory tract infection, and oxygen requirement are all associated with increased overall mortality [21][25][26][27].
5. Diagnostic Tools
References
- Barriga, F.; Ramirez, P.; Wietstruck, A.; Rojas, N. Hematopoietic stem cell transplantation: Clinical use and perspectives. Biol. Res. 2012, 45, 307–316.
- Cordonnier, C.; Bernaudin, J.F.; Bierling, P.; Huet, Y.; Vernant, J.P. Pulmonary complications occurring after allogeneic bone marrow transplantation. A study of 130 consecutive transplanted patients. Cancer 1986, 58, 1047–1054.
- Jules-Elysee, K.; Stover, D.E.; Yahalom, J.; White, D.A.; Gulati, S.C. Pulmonary complications in lymphoma patients treated with high-dose therapy autologous bone marrow transplantation. Am. Rev. Respir Dis. 1992, 146, 485–491.
- Shorr, A.F.; Moores, L.K.; Edenfield, W.J.; Christie, R.J.; Fitzpatrick, T.M. Mechanical ventilation in hematopoietic stem cell transplantation: Can We effectively predict outcomes? Chest 1999, 116, 1012–1018.
- Forman, S.J.; Negrin, R.S.; Antin, J.H.; Appelbaum, F.R. Thomas’ Hematopoietic Cell Transplantation; Wiley Blackwell: Hoboken, NJ, USA, 2016.
- Copelan, E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006, 354, 1813–1826.
- Majhail, N.S.; Farnia, S.H.; Carpenter, P.A.; Champlin, R.E.; Crawford, S.; Marks, D.I.; Omel, J.L.; Orchard, P.J.; Palmer, J.; Saber, W.; et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1863–1869.
- Spitzer, T.R. Engraftment syndrome: Double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015, 50, 469–475.
- Bazinet, A.; Popradi, G. A general practitioner’s guide to hematopoietic stem-cell transplantation. Curr. Oncol. 2019, 26, 187–191.
- Quesenberry, P.J.; Stewart, F.M.; Becker, P.; D’Hondt, L.; Frimberger, A.; Lambert, J.F.; Colvin, G.A.; Miller, C.; Heyes, C.; Abedi, M.; et al. Stem cell engraftment strategies. Ann. N. Y. Acad Sci. 2001, 938, 54–61.
- Spitzer, T.R. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 27, 893–898.
- Azar, M.M.; Gaston, D.C.; Kotton, C.N.; Malinis, M.F. Emerging Microbiology Diagnostics for Transplant Infections: On the Cusp of a Paradigm Shift. Transplantation 2020, 104, 1358–1384.
- Dykewicz, C.A. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2001, 33, 139–144.
- Omrani, A.S.; Almaghrabi, R.S. Complications of hematopoietic stem cell transplantation: Bacterial infections. Hematol. Oncol. Stem Cell Ther. 2017, 10, 228–232.
- Hatzimichael, E.; Tuthill, M. Hematopoietic stem cell transplantation. Stem Cells Cloning 2010, 3, 105–117.
- Chuzi, S.; Tavora, F.; Cruz, M.; Costa, R.; Chaet, Y.K.; A Carneiro, B.; Giles, F.J. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag. Res. 2017, 9, 207–213.
- Limper, A.H.; Knox, K.S.; Sarosi, G.A.; Ampel, N.M.; Bennett, J.E.; Catanzaro, A.; Davies, S.F.; Dismukes, W.E.; Hage, C.A.; Marr, K.A.; et al. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 2011, 183, 96–128.
- Lanternier, F.; Sun, H.-Y.; Ribaud, P.; Singh, N.; Kontoyiannis, D.P.; Lortholary, O. Mucormycosis in Organ and Stem Cell Transplant Recipients. Clin. Infect. Dis. 2012, 54, 1–8.
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.; Lee, S.-O.; Choi, S.-H.; Kim, Y.; Woo, J.; Kim, S.-H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18.
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingalrd, J.R.; Young, J.-A.; Boeckh, M.A. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238.
- Shah, D.P.; Ghantoji, S.S.; Shah, J.N.; El Taoum, K.K.; Jiang, Y.; Popat, U.; Hosing, C.; Rondon, G.; Tarrand, J.J.; Champlin, R.E.; et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J. Antimicrob. Chemother. 2013, 68, 1872–1880.
- Kim, Y.J.; Guthrie, K.A.; Waghmare, A.; Walsh, E.E.; Falsey, A.R.; Kuypers, J.; Cent, A.; Etnglund, J.A.; Boeckh, M. Respiratory syncytial virus in hematopoietic cell transplant recipients: Factors determining progression to lower respiratory tract disease. J. Infect. Dis. 2014, 209, 1195–1204.
- Ljungman, P.; Ward, K.N.; Crooks, B.N.; Parker, A.; Martino, R.; Shaw, P.; Brinch, L.; Brune, M.; De La Camara, R.; Dekker, A.W.; et al. Respiratory virus infections after stem cell transplantation: A prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001, 28, 479–484.
- Pilie, P.; Werbel, W.A.; Riddell Jt Shu, X.; Schaubel, D.; Gregg, K.S. Adult patients with respiratory syncytial virus infection: Impact of solid organ and hematopoietic stem cell transplantation on outcomes. Transpl. Infect. Dis. 2015, 17, 551–557.
- Renaud, C.; Xie, H.; Seo, S.; Kuypers, J.; Cent, A.; Corey, L.; Leisenring, W.; Boeckh, M.; Etnglund, J.A. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol. Blood Marrow Transplant. 2013, 19, 1220–1226.
- McCarthy, A.J.; Kingman, H.M.; Kelly, C.; Taylor, G.S.; O Caul, E.; Grier, D.; Moppett, J.; Foot, A.B.M.; Cornish, J.M.; Oakhill, A.; et al. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation. Bone Marrow Transplant. 1999, 24, 1315–1322.
- Seo, S.; Campbell, A.P.; Xie, H.; Chien, J.W.; Leisenring, W.; Etnglund, J.A.; Boeckh, M. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: Significance of stem cell source and oxygen requirement. Biol. Blood Marrow Transplant. 2013, 19, 589–596.
- Sakata, K.K.; Klassen, C.L.; Bollin, K.B.; Grys, T.E.; Slack, J.L.; Wesselius, L.J.; Vikra, H.R. Microbiologic yield of bronchoalveolar lavage specimens from stem cell transplant recipients. Transpl. Infect. Dis. 2017, 19, e12684.
- Wahla, A.S.; Chatterjee, A.; Khan, I.I.; Conforti, J.F.; Haponik, E. Survey of academic pulmonologists, oncologists, and infectious disease physicians on the role of bronchoscopy in managing hematopoietic stem cell transplantation patients with pulmonary infiltrates. J. Bronchol. Interv. Pulmonol. 2014, 21, 32–39.
- Shannon, V.R.; Andersson, B.S.; Lei, X.; Champlin, R.E.; Kontoyiannis, D.P. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010, 45, 647–655.
- Jorge, L.; Torres, D.; Languasco, A.; Rodriguez, P.; Bonvehí, P.; Temporiti, E.; Relloso, S.; Videlal, C.; Herrera, F. Clinical Usefulness of Bronchoalveolar Lavage in the Management of Pulmonary Infiltrates in Adults with Hematological Malignancies and Stem Cell Transplantation. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020025.
- Feinstein, M.B.; Habtes, I.; Giralt, S.; Stover, D.E. Utility of Bronchoscopy with Bronchoalveolar Lavage among Hematologic Transplant Recipients in the Era of Noninvasive Testing. Respiration 2021, 100, 339–346.
- Harris, B.; Lowy, F.D.; Stover, D.E.; Arcasoy, S.M. Diagnostic bronchoscopy in solid-organ and hematopoietic stem cell transplantation. Ann. Am. Thorac. Soc. 2013, 10, 39–49.
- Gilbert, C.R.; Lerner, A.; Baram, M.; Awsare, B.K. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—A single center fourteen year experience. Arch. Bronconeumol. 2013, 49, 189–195.
- Mulabecirovic, A.; Gaulhofer, P.; Auner, H.W.; Popper, H.; Krause, R.; Hesse, C.; Sill, H. Pulmonary infiltrates in patients with haematologic malignancies: Transbronchial lung biopsy increases the diagnostic yield with respect to neoplastic infiltrates and toxic pneumonitis. Ann. Hematol. 2004, 83, 420–422.
- O’Dwyer, D.N.; Duvall, A.S.; Xia, M.; Hoffman, T.C.; Bloye, K.S.; Bulte, C.A.; Zhou, X.; Murray, S.; Moore, B.; Yanik, G.A. Transbronchial biopsy in the management of pulmonary complications of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018, 53, 193–198.
- Ikezawa, Y.; Shinagawa, N.; Sukoh, N.; Morimoto, M.; Kikuchi, H.; Watanabe, M.; Nakano, K.; Oizumi, S.; Nishimura, M. Usefulness of Endobronchial Ultrasonography With a Guide Sheath and Virtual Bronchoscopic Navigation for Ground-Glass Opacity Lesions. Ann. Thorac. Surg. 2017, 103, 470–475.
- Hayama, M.; Okamoto, N.; Suzuki, H.; Tamiya, M.; Shiroyama, T.; Tanaka, A.; Nishida, T.; Nishihara, T.; Uehara, N.; Morishita, N.; et al. Radial endobronchial ultrasound with a guide sheath for diagnosis of peripheral cavitary lung lesions: A retrospective study. BMC Pulm. Med. 2016, 16, 76.
- Bouso, J.M.; Yendur, O.; Hysinger, E.; Planet, P.J.; Haas, A.; Goldfarb, S.; Piccione, J. Endobronchial Ultrasound–guided Biopsy Is Feasible, Safe, and Improves Diagnostic Yields in Immunocompromised Children. Am. J. Respir. Crit. Care Med. 2019, 201, 384–386.
- Harris, B.; Geyer, A.I. Diagnostic Evaluation of Pulmonary Abnormalities in Patients with Hematologic Malignancies and Hematopoietic Cell Transplantation. Clin. Chest Med. 2017, 38, 317–331.
- Carrafiello, G.; Laganà, D.; Nosari, A.M.; Guffanti, C.; Morra, E.; Recaldini, C.; D’Alba, M.J.; Sonvico, U.; Vanzulli, A.; Fugazzola, C. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol. Med. 2006, 111, 33–41.
- Snyder, C.L.; Ramsay, N.K.; McGlave, P.B.; Ferrell, K.L.; Leonard, A.S. Diagnostic open-lung biopsy after bone marrow transplantation. J. Pediatr. Surg. 1990, 25, 871–876.
- Chellapandian, D.; Lehrnbecher, T.; Phillips, B.; Fisher, B.T.; Zaoutis, T.E.; Steinbach, W.J.; Beyene, J.; Sung, L. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 501–509.
- Wingard, J.R.; Hiemenz, J.W.; Jantz, M.A. How I manage pulmonary nodular lesions and nodular infiltrates in patients with hematologic malignancies or undergoing hematopoietic cell transplantation. Blood 2012, 120, 1791–1800.




