
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tala Abu Samaan | + 5064 word(s) | 5064 | 2021-07-22 08:41:25 | | | |
| 2 | Vivi Li | Meta information modification | 5064 | 2021-08-05 03:17:07 | | |
Video Upload Options
Paclitaxel (PTX), the most widely used anticancer drug, is applied for the treatment of various types of malignant diseases. Mechanisms of PTX action represent several ways in which PTX affects cellular processes resulting in programmed cell death. PTX is frequently used as the first-line treatment drug in breast cancer (BC). Unfortunately, the resistance of BC to PTX treatment is a great obstacle in clinical applications and one of the major causes of death associated with treatment failure. Factors contributing to PTX resistance, such as ABC transporters, microRNAs (miRNAs), or mutations in certain genes, along with side effects of PTX including peripheral neuropathy or hypersensitivity associated with the vehicle used to overcome its poor solubility, are responsible for intensive research concerning the use of PTX in preclinical and clinical studies. Novelties such as albumin-bound PTX (nab-PTX) demonstrate a progressive approach leading to higher efficiency and decreased risk of side effects after drug administration. Moreover, PTX nanoparticles for targeted treatment of BC promise a stable and efficient therapeutic intervention.
1. Introduction
2. Paclitaxel: Fundamental Drug in Chemotherapy and Novel Advances in its Application
2.1. The Origin of Paclitaxel
2.2. Paclitaxel’s Mechanism of Action
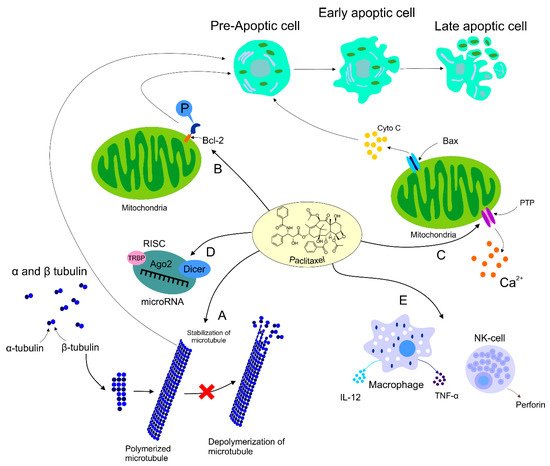
2.3. Paclitaxel’s Effect on HER2+ Breast Cancer
2.4. Dose Ranges Administered
| Condition | Administration Schedule | Concentration Range | Reference |
|---|---|---|---|
| Adjuvant therapy with doxorubicin (node-positive or high-risk node-negative BC) | Every 3 weeks | 175 mg/m2 IV perfusion over 3 h (4 courses) | [57] |
| Weekly | 80 mg/m2 IV perfusion over 1 h (12 courses) | [58] | |
| Failure of neoadjuvant therapy (MBC or relapse within 6 months of neoadjuvant therapy) | Every 3 weeks | 175 mg/m2 IV perfusion over 3 h | [57] |
| Untreated MBC | Every 3 weeks (max. of 8 cycles) | 200 mg/m2 IV infusion over 3 h + total dose of 480 mg/m2 doxorubicin 25 mg oral prednisone pre-treatment (12 h before treatment) 10 mg intramuscular chlorpheniramine + 300 mg intravenous cimetidine (both 30 min before PTX) |
[59] |
| Neoadjuvant Drug Combination | Patient Eligibility | Concentration Range | Efficacy | Reference |
|---|---|---|---|---|
| PTX after Doxorubicin + Cyclophosphamide | Node-positive BC with resected adenocarcinoma | 60 mg/m2 doxorubicin + 600 mg/m2 cyclophosphamide (IV infusion for 30 min to 2 h every 21 days, −4 cycles +4 cycles of 225 mg/m2 PTX (day 1 of each cycle) | PTX + doxorubicin + cyclophosphamide: ↑ DFS by 17% Acceptable toxicity |
[61] |
| PTX + Bevacizumab | MBC patients with/without previous hormonal therapy or adjuvant chemotherapy | 90 mg/m2 PTX (day 1, 8, 15 every 4 weeks) + 10 mg/kg (day 1 and 15) | ↑ progression-free survival (in comparison to PTX alone) ↑ frequency of hypertension, proteinuria, headache, cerebrospinal ischemia |
[62] |
| PTX + Ttrastuzumab | Breast adenocarcinoma patients (tumor no larger than 3 cm, node-negative, min. LVEF of 50%, adequate hematopoietic and liver function) | 80 mg/m2 PTX for 12 weeks + 4 mg/kg trastuzumab (day 1) → 2 mg/kg weekly (12 doses) | 98.7% disease-free survival 99.2% 3-year rate of recurrence-free survival (95%CI) 2.92% of patients reported adverse effects |
[63] |
| PTX + Trastuzumab then post-operative Doxorubicin + Cyclophosphamide | Stage II or III BC patients | Dexamethasone pretreatment (20 mg) + diphenhydramine (12 and 6 h before treatment) and H2-blocker (50 mg) Trastuzumab (one-time loading dose 4 mg/kg) → weekly 2 mg/kg IV infusion for 11 weeks + 175 mg/m2 of IV PTX over 3 h (every 3 weeks, 4 cycles) 2–5 weeks post-op: doxorubicin + cyclophosphamide |
75% clinical response with 18% complete pathologic response Stage 3 tumors responded more than stage 2 tumors |
[64] |
| PTX + rhG-CSF | BC patients (last radiation therapy at least 4 weeks prior to chemotherapy) | 250 mg/m2 of IV PTX (for 24 h every 21 days, dose adjusted to granulocyte and platelet nadirs) 5 μg/kg/d of rhG-CSF (subcutaneously on day 3 through 10/cycle) |
CR—12% of patients PR—50% of patients Inverse correlation between response and median age of patients Minimal toxic effects |
[65] |
2.5. Breast Tumor Resistance to Paclitaxel
2.6. Paclitaxel’s Side Effects
2.7. Albumin-Bound Paclitaxel and Comparison with Its Conventional Alternative
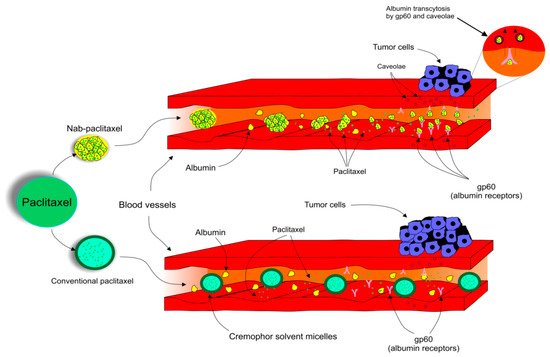
3. Novel Insights into the Application of Paclitaxel in Clinical Practice
References
- Clatici, V.G.; Voicu, C.; Voaides, C.; Roseanu, A.; Icriverzi, M.; Jurcoane, S. Diseases of civilization—Cancer, diabetes, obesity and acne—The implication of milk, IGF-1 and mTORC1. Mædica 2018, 13, 273–281.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953.
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent advances in the treatment of breast cancer. Front. Oncol. 2018, 8, 227.
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 101, 458–477.
- Vici, P.; Viola, G.; Rossi, S.; Botti, C.; Vitucci, C.; Sergi, D.; Ferranti, F.R.; Saracca, E.; Di Lauro, L.; Corsetti, S.; et al. Optimal sequence of anthracyclines and taxanes as adjuvant breast cancer treatment. Clin. Ter. 2008, 159, 453–456.
- Kellokumpu-Lehtinen, P.; Tuunanen, T.; Asola, R.; Elomaa, L.; Heikkinen, M.; Kokko, R.; Järvenpää, R.; Lehtinen, I.; Maiche, A.; Kaleva-Kerola, J.; et al. Weekly paclitaxel—An effective treatment for advanced breast cancer. Anticancer Res. 2013, 33, 2623–2627.
- Van Vuuren, R.J.; Visagie, M.H.; Theron, A.E.; Joubert, A.M. Antimitotic drugs in the treatment of cancer. Cancer Chemother. Pharmacol. 2015, 76, 1101–1112.
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta 2008, 1785, 96–132.
- Matsuyoshi, S.; Shimada, K.; Nakamura, M.; Ishida, E.; Konishi, N. Bcl-2 phosphorylation has pathological significance in human breast cancer. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2006, 73, 205–212.
- Pan, Z.; Avila, A.; Gollahon, L. Paclitaxel induces apoptosis in breast cancer cells through different calcium—Regulating mechanisms depending on external calcium conditions. Int. J. Mol. Sci. 2014, 15, 2672–2694.
- Wanderley, C.W.; Colon, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1- profile in a TLR4-dependent manner. Cancer Res. 2018, 78, 5891–5900.
- Panis, C.; Pavanelli, W.R. Cytokines as mediators of pain-related process in breast cancer. Mediators Inflamm. 2015, 2015, 129034.
- Sudo, T.; Nitta, M.; Saya, H.; Ueno, N.T. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004, 64, 2502–2508.
- Chen, J.; Tian, W.; He, H.; Chen, F.; Huang, J.; Wang, X.; Chen, Z. Downregulation of miR-200c-3p contributes to the resistance of breast cancer cells to paclitaxel by targeting SOX2. Oncol. Rep. 2018, 40, 3821–3829.
- Xu, R.; Sato, N.; Yanai, K.; Akiyoshi, T.; Nagai, S.; Wada, J.; Koga, K.; Mibu, R.; Nakamura, M.; Katano, M. Enhancement of Paclitaxel-induced apoptosis by inhibition of mitogen-activated protein Kinase pathway in colon cancer cells. Anticancer Res. 2009, 29, 261–270.
- Mahtani, R.L.; Parisi, M.; Glück, S.; Ni, Q.; Park, S.; Pelletier, C.; Faria, C.; Braiteh, F. Comparative effectiveness of early-line nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer: A US community-based real-world analysis. Cancer Manag. Res. 2018, 10, 249–256.
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681.
- Walsh, V.; Goodman, J. From taxol to Taxol: The changing identities and ownership of an anti-cancer drug. Med. Anthropol. 2002, 21, 307–336.
- Kumar, P.; Raza, K.; Kaushik, L.; Malik, R.; Arora, S.; Katare, O.P. Role of colloidal drug delivery carriers in Taxane-mediated chemotherapy: A review. Curr. Pharm. Des. 2016, 22, 5127–5143.
- Perdue, R.E.; Hartwell, J.T. Search for plant sources of anticancer drugs. Morris Arbor. Bull 1969, 20, 35–53.
- Walsh, V.; Goodman, J. Cancer chemotherapy, biodiversity, public and private property: The case of the anti-cancer drug taxol. Soc. Sci. Med. 1982 1999, 49, 1215–1225.
- Holton, R.A.; Kim, H.B.; Somoza, C.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Shindo, M.; Smith, C.C.; Kim, S. First total synthesis of taxol. 2. Completion of the C and D rings. J. Am. Chem. Soc. 1994, 116, 1599–1600.
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216.
- Sackett, D.; Fojo, T. Taxanes. Cancer Chemother. Biol. Response Modif. 1997, 17, 59–79.
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667.
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A novel investigational antimicrotubule agent. JNCI J. Natl. Cancer Inst. 1990, 82, 1247–1259.
- Collins, C.A.; Vallee, R.B. Temperature-dependent reversible assembly of taxol-treated microtubules. J. Cell Biol. 1987, 105, 2847–2854.
- De Brabander, M.; Geuens, G.; Nuydens, R.; Willebrords, R.; De Mey, J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc. Natl. Acad. Sci. USA 1981, 78, 5608–5612.
- Jordan, M.A.; Toso, R.J.; Thrower, D.; Wilson, L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA 1993, 90, 9552–9556.
- Jordan, M.A.; Wilson, L. Microtubules and actin filaments: Dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 1998, 10, 123–130.
- Long, B.H.; Fairchild, C.R. Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telephase. Cancer Res. 1994, 54, 4355–4361.
- Giannakakou, P.; Robey, R.; Fojo, T.; Blagosklonny, M.V. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: Molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 2001, 20, 3806–3813.
- Tran, T.-A.; Gillet, L.; Roger, S.; Besson, P.; White, E.; Le Guennec, J.-Y. Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness. Biochem. Biophys. Res. Commun. 2009, 379, 304–308.
- Shetti, D.; Zhang, B.; Fan, C.; Mo, C.; Lee, B.H.; Wei, K. Low dose of paclitaxel combined with XAV939 attenuates metastasis, angiogenesis and growth in breast cancer by suppressing Wnt signaling. Cells 2019, 8, 892.
- Blagosklonny, M.V.; Giannakakou, P.; el-Deiry, W.S.; Kingston, D.G.; Higgs, P.I.; Neckers, L.; Fojo, T. Raf-1/bcl-2 phosphorylation: A step from microtubule damage to cell death. Cancer Res. 1997, 57, 130–135.
- Haldar, S.; Basu, A.; Croce, C.M. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997, 57, 229–233.
- Ling, Y.H.; Yang, Y.; Tornos, C.; Singh, B.; Perez-Soler, R. Paclitaxel-induced apoptosis is associated with expression and activation of c-Mos gene product in human ovarian carcinoma SKOV3 cells. Cancer Res. 1998, 58, 3633–3640.
- Blagosklonny, M.V.; Fojo, T. Molecular effects of paclitaxel: Myths and reality (a critical review). Int. J. Cancer 1999, 83, 151–156.
- Haldar, S.; Chintapalli, J.; Croce, C.M. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996, 56, 1253–1255.
- Ibrado, A.M.; Liu, L.; Bhalla, K. Bcl-xL overexpression inhibits progression of molecular events leading to paclitaxel-induced apoptosis of human acute myeloid leukemia HL-60 cells. Cancer Res. 1997, 57, 1109–1115.
- Blajeski, A.L.; Kottke, T.J.; Kaufmann, S.H. A multistep model for Paclitaxel-induced apoptosis in human breast cancer cell lines. Exp. Cell Res. 2001, 270, 277–288.
- Ofir, R.; Seidman, R.; Rabinski, T.; Krup, M.; Yavelsky, V.; Weinstein, Y.; Wolfson, M. Taxol-induced apoptosis in human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3 and caspase-9 independent. Cell Death Differ. 2002, 9, 636–642.
- Varbiro, G.; Veres, B.; Gallyas, F.; Sumegi, B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic. Biol. Med. 2001, 31, 548–558.
- Kidd, J.F.; Pilkington, M.F.; Schell, M.J.; Fogarty, K.E.; Skepper, J.N.; Taylor, C.W.; Thorn, P. Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J. Biol. Chem. 2002, 277, 6504–6510.
- Asghari, F.; Haghnavaz, N.; Shanehbandi, D.; Khaze, V.; Baradaran, B.; Kazemi, T. Differential altered expression of let-7a and miR-205 tumor-suppressor miRNAs in different subtypes of breast cancer under treatment with Taxol. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 941–945.
- Tao, W.-Y.; Liang, X.-S.; Liu, Y.; Wang, C.-Y.; Pang, D. Decrease of let-7f in low-dose metronomic paclitaxel chemotherapy contributed to upregulation of thrombospondin-1 in breast cancer. Int. J. Biol. Sci. 2015, 11, 48–58.
- Javeed, A.; Ashraf, M.; Riaz, A.; Ghafoor, A.; Afzal, S.; Mukhtar, M.M. Paclitaxel and immune system. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 38, 283–290.
- Larionova, I.; Cherdyntseva, N.; Liu, T.; Patysheva, M.; Rakina, M.; Kzhyshkowska, J. Interaction of tumor-associated macrophages and cancer chemotherapy. OncoImmunology 2019, 8, e1596004.
- Emens, L.A.; Jaffee, E.M. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005, 65, 8059–8064.
- John, J.; Ismail, M.; Riley, C.; Askham, J.; Morgan, R.; Melcher, A.; Pandha, H. Differential effects of Paclitaxel on dendritic cell function. BMC Immunol. 2010, 11, 14.
- Kubo, M.; Morisaki, T.; Matsumoto, K.; Tasaki, A.; Yamanaka, N.; Nakashima, H.; Kuroki, H.; Nakamura, K.; Nakamura, M.; Katano, M. Paclitaxel probably enhances cytotoxicity of natural killer cells against breast carcinoma cells by increasing perforin production. Cancer Immunol. Immunother. CII 2005, 54, 468–476.
- Yu, D.; Jing, T.; Liu, B.; Yao, J.; Tan, M.; McDonnell, T.J.; Hung, M.C. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol. Cell 1998, 2, 581–591.
- Hayes, D.F.; Thor, A.D.; Dressler, L.G.; Weaver, D.; Edgerton, S.; Cowan, D.; Broadwater, G.; Goldstein, L.J.; Martino, S.; Ingle, J.N.; et al. HER2 and response to paclitaxel in node-positive breast cancer. N. Engl. J. Med. 2007, 357, 1496–1506.
- Baselga, J.; Seidman, A.D.; Rosen, P.P.; Norton, L. HER2 overexpression and paclitaxel sensitivity in breast cancer: Therapeutic implications. Oncol. Williston Park N Y 1997, 11 (Suppl. 2), 43–48.
- Blagosklonny, M.V.; Schulte, T.W.; Nguyen, P.; Mimnaugh, E.G.; Trepel, J.; Neckers, L. Taxol induction of p21WAF1 and p53 requires c-raf-1. Cancer Res. 1995, 55, 4623–4626.
- Winer, E.P.; Berry, D.A.; Woolf, S.; Duggan, D.; Kornblith, A.; Harris, L.N.; Michaelson, R.A.; Kirshner, J.A.; Fleming, G.F.; Perry, M.C.; et al. Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: cancer and leukemia group B trial 9342. J. Clin. Oncol. 2004, 22, 2061–2068.
- Spielmann, M. Taxol (Paclitaxel) in patients with metastatic breast carcinoma who have failed prior chemotherapy: Interim results of a multinational study. Oncology 1994, 51, 25–28.
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671.
- Gianni, L.; Munzone, E.; Capri, G.; Fulfaro, F.; Tarenzi, E.; Villani, F.; Spreafico, C.; Laffranchi, A.; Caraceni, A.; Martini, C. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 2688–2699.
- Seidman, A.D.; Hudis, C.A.; Albanell, J.; Albanel, J.; Tong, W.; Tepler, I.; Currie, V.; Moynahan, M.E.; Theodoulou, M.; Gollub, M.; et al. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 3353–3361.
- Mamounas, E.P.; Bryant, J.; Lembersky, B.; Fehrenbacher, L.; Sedlacek, S.M.; Fisher, B.; Wickerham, D.L.; Yothers, G.; Soran, A.; Wolmark, N. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3686–3696.
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679.
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant Paclitaxel and Trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 2015, 372, 134–141.
- Burstein, H.J.; Harris, L.N.; Gelman, R.; Lester, S.C.; Nunes, R.A.; Kaelin, C.M.; Parker, L.M.; Ellisen, L.W.; Kuter, I.; Gadd, M.A.; et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: A pilot study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 46–53.
- Reichman, B.S.; Seidman, A.D.; Crown, J.P.; Heelan, R.; Hakes, T.B.; Lebwohl, D.E.; Gilewski, T.A.; Surbone, A.; Currie, V.; Hudis, C.A. Paclitaxel and recombinant human granulocyte colony-stimulating factor as initial chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1993, 11, 1943–1951.
- Chi, Y.; Xue, J.; Huang, S.; Xiu, B.; Su, Y.; Wang, W.; Guo, R.; Wang, L.; Li, L.; Shao, Z.; et al. CapG promotes resistance to paclitaxel in breast cancer through transactivation of PIK3R1/P50. Theranostics 2019, 9, 6840–6855.
- Wang, H.; Vo, T.; Hajar, A.; Li, S.; Chen, X.; Parissenti, A.M.; Brindley, D.N.; Wang, Z. Multiple mechanisms underlying acquired resistance to taxanes in selected docetaxel-resistant MCF-7 breast cancer cells. BMC Cancer 2014, 14, 37.
- Wang, Y.; Zhou, Y.; Zheng, Z.; Li, J.; Yan, Y.; Wu, W. Sulforaphane metabolites reduce resistance to paclitaxel via microtubule disruption. Cell Death Dis. 2018, 9, 1134.
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228.
- Childs, S.; Ling, V. The MDR superfamily of genes and its biological implications. Important Adv. Oncol. 1994, 21–36.
- Yang, N.; Wang, C.; Wang, J.; Wang, Z.; Huang, D.; Yan, M.; Kamran, M.; Liu, Q.; Xu, B. Aurora kinase A stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J. Cell. Mol. Med. 2019, 23, 6442–6453.
- Khongkow, P.; Gomes, A.R.; Gong, C.; Man, E.P.S.; Tsang, J.W.-H.; Zhao, F.; Monteiro, L.J.; Coombes, R.C.; Medema, R.H.; Khoo, U.S.; et al. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene 2016, 35, 990–1002.
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320.
- Magee, P.; Shi, L.; Garofalo, M. Role of microRNAs in chemoresistance. Ann. Transl. Med. 2015, 3, 332.
- Luo, Y.; Hua, T.; You, X.; Lou, J.; Yang, X.; Tang, N. Effects of MiR-107 on the chemo-drug sensitivity of breast cancer cells. Open Med. 2019, 14, 59–65.
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin. Epigenetics 2016, 8, 115.
- Lv, K.; Liu, L.; Wang, L.; Yu, J.; Liu, X.; Cheng, Y.; Dong, M.; Teng, R.; Wu, L.; Fu, P.; et al. Lin28 mediates paclitaxel resistance by modulating p21, Rb and Let-7a miRNA in breast cancer cells. PLoS ONE 2012, 7, e40008.
- Song, Y.-K.; Wang, Y.; Wen, Y.-Y.; Zhao, P.; Bian, Z.-J. MicroRNA-22 suppresses breast cancer cell growth and increases Paclitaxel sensitivity by targeting NRAS. Technol. Cancer Res. Treat. 2018, 17.
- Lasham, A.; Mehta, S.Y.; Fitzgerald, S.J.; Woolley, A.G.; Hearn, J.I.; Hurley, D.G.; Ruza, I.; Algie, M.; Shelling, A.N.; Braithwaite, A.W.; et al. A novel EGR-1 dependent mechanism for YB-1 modulation of paclitaxel response in a triple negative breast cancer cell line: EGR-1-dependent mechanism for YB-1 modulation. Int. J. Cancer 2016, 139, 1157–1170.
- Nestal de Moraes, G.; Ji, Z.; Fan, L.Y.-N.; Yao, S.; Zona, S.; Sharrocks, A.D.; Lam, E.W.-F. SUMOylation modulates FOXK2-mediated paclitaxel sensitivity in breast cancer cells. Oncogenesis 2018, 7, 29.
- Zhang, D.; Yang, R.; Wang, S.; Dong, Z. Paclitaxel: new uses for an old drug. Drug Des. Devel. Ther. 2014, 8, 279–284.
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621.
- Yamamoto, Y.; Kawano, I. Iwase Nab-paclitaxel for the treatment of breast cancer: Efficacy, safety, and approval. Onco Targets Ther. 2011, 4, 123–136.
- Ma, P.; Mumper, R.J. Paclitaxel nano-delivery systems: A comprehensive review. J. Nanomed. Nanotechnol. 2013, 4, 1000164.
- Vishnu, P.; Roy, V. Safety and efficacy of Nab-Paclitaxel in the treatment of patients with breast cancer. Breast Cancer Basic Clin. Res. 2011, 5, 53–65.
- Walker, F.E. Paclitaxel (TAXOL®): Side effects and patient education issues. Semin. Oncol. Nurs. 1993, 9, 6–10.
- Vahdat, L.; Papadopoulos, K.; Lange, D.; Leuin, S.; Kaufman, E.; Donovan, D.; Frederick, D.; Bagiella, E.; Tiersten, A.; Nichols, G.; et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 1192–1197.
- Flatters, S.J.L.; Bennett, G.J. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161.
- Ta-Chung, C.; Zyting, C.; Ling-Ming, T.; Tzeon-Jye, C.; Ruey-Kuen, H.; Wei-Shu, W.; Chueh-Chuan, Y.; Muh-Hwa, Y.; Liang-Tsai, H.; Jin-Hwang, L.; et al. Paclitaxel in a novel formulation containing less Cremophor EL as first-line therapy for advanced breast cancer: A phase II trial. Investig. New Drugs 2005, 23, 171–177.
- Rowinsky, E.K.; McGuire, W.P.; Guarnieri, T.; Fisherman, J.S.; Christian, M.C.; Donehower, R.C. Cardiac disturbances during the administration of taxol. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 1704–1712.
- Bristow, M.R.; Sageman, W.S.; Scott, R.H.; Billingham, M.E.; Bowden, R.E.; Kernoff, R.S.; Snidow, G.H.; Daniels, J.R. Acute and chronic cardiovascular effects of doxorubicin in the dog: The cardiovascular pharmacology of drug-induced histamine release. J. Cardiovasc. Pharmacol. 1980, 2, 487–515.
- Hoffman, R.M.; Bouvet, M. Nanoparticle albumin-bound-paclitaxel: A limited improvement under the current therapeutic paradigm of pancreatic cancer. Expert Opin. Pharmacother. 2015, 16, 943–947.
- Roy, V.; LaPlant, B.R.; Gross, G.G.; Bane, C.L.; Palmieri, F.M.; North Central Cancer Treatment Group. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane) in combination with gemcitabine in patients with metastatic breast cancer (N0531). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 449–453.
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1317–1324.
- Iglesias, J. Nab-Paclitaxel (Abraxane®): An albumin-bound cytotoxic exploiting natural delivery mechanisms into tumors. Breast Cancer Res. BCR 2009, 11, S21.
- Aapro, M.S.; Von Minckwitz, G. Molecular basis for the development of novel taxanes in the treatment of metastatic breast cancer. Eur. J. Cancer Suppl. 2008, 6, 3–11.
- Zhao, Y.; Lv, F.; Chen, S.; Wang, Z.; Zhang, J.; Zhang, S.; Cao, J.; Wang, L.; Cao, E.; Wang, B.; et al. Caveolin-1 expression predicts efficacy of weekly nab-paclitaxel plus gemcitabine for metastatic breast cancer in the phase II clinical trial. BMC Cancer 2018, 18, 1019.
- Zhu, A.; Yuan, P.; Du, F.; Hong, R.; Ding, X.; Shi, X.; Fan, Y.; Wang, J.; Luo, Y.; Ma, F.; et al. SPARC overexpression in primary tumors correlates with disease recurrence and overall survival in patients with triple negative breast cancer. Oncotarget 2016, 7, 76628–76634.
- Desai, N.; Trieu, V.; Damascelli, B.; Soon-Shiong, P. SPARC expression correlates with tumor response to albumin-bound Paclitaxel in head and neck cancer patients. Transl. Oncol. 2009, 2, 59–64.
- Blum, J.L.; Savin, M.A.; Edelman, G.; Pippen, J.E.; Robert, N.J.; Geister, B.V.; Kirby, R.L.; Clawson, A.; O’Shaughnessy, J.A. Phase II study of weekly albumin-bound Paclitaxel for patients with metastatic breast cancer heavily pretreated with Taxanes. Clin. Breast Cancer 2007, 7, 850–856.
- Montana, M.; Ducros, C.; Verhaeghe, P.; Terme, T.; Vanelle, P.; Rathelot, P. Albumin-bound Paclitaxel: The benefit of this new formulation in the treatment of various cancers. J. Chemother. 2011, 23, 59–66.
- Matsui, A.; Tatibana, A.; Suzuki, N.; Hirata, M.; Oishi, Y.; Hamaguchi, Y.; Murata, Y.; Nagayama, A.; Iwata, Y.; Okamoto, Y. Evaluation of efficacy and safety of upfront weekly nanoparticle albumin-bound Paclitaxel for HER2-negative breast cancer. Anticancer Res. 2017, 37, 6481–6488.
- Martín, M.; Chacón, J.I.; Antón, A.; Plazaola, A.; García-Martínez, E.; Seguí, M.A.; Sánchez-Rovira, P.; Palacios, J.; Calvo, L.; Esteban, C.; et al. Neoadjuvant therapy with weekly nanoparticle albumin-bound Paclitaxel for luminal early breast cancer patients: Results from the NABRAX study (GEICAM/2011-02), a multicenter, non-randomized, phase II trial, with a companion biomarker analysis. Oncologist 2017, 22, 1301–1308.
- Takashima, T.; Kawajiri, H.; Nishimori, T.; Tei, S.; Nishimura, S.; Yamagata, S.; Tokunaga, S.; Mizuyama, Y.; Sunami, T.; Tezuka, K.; et al. Safety and efficacy of low-dose nanoparticle albumin-bound Paclitaxel for HER2-negative metastatic breast cancer. Anticancer Res. 2018, 38, 379–383.
- Ibrahim, N.K.; Samuels, B.; Page, R.; Doval, D.; Patel, K.M.; Rao, S.C.; Nair, M.K.; Bhar, P.; Desai, N.; Hortobagyi, G.N. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J. Clin. Oncol. 2005, 23, 6019–6026.
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound Paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803.
- Gianni, L.; Mansutti, M.; Anton, A.; Calvo, L.; Bisagni, G.; Bermejo, B.; Semiglazov, V.; Thill, M.; Chacon, J.I.; Chan, A.; et al. Comparing neoadjuvant nab-Paclitaxel vs. Paclitaxel both followed by Anthracycline regimens in women with ERBB2/HER2-negative breast cancer-the Evaluating Treatment With Neoadjuvant Abraxane (ETNA) trial: A randomized phase 3 clinical trial. JAMA Oncol. 2018, 4, 302–308.
- Feng, Z.-Q.; Yan, K.; Li, J.; Xu, X.; Yuan, T.; Wang, T.; Zheng, J. Magnetic Janus particles as a multifunctional drug delivery system for paclitaxel in efficient cancer treatment. Mater. Sci. Eng. C 2019, 104, 110001.
- Zhang, Q.; Zhao, J.; Hu, H.; Yan, Y.; Hu, X.; Zhou, K.; Xiao, S.; Zhang, Y.; Feng, N. Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int. J. Pharm. 2019, 569, 118595.
- Efferth, T.; Saeed, M.E.M.; Mirghani, E.; Alim, A.; Yassin, Z.; Saeed, E.; Khalid, H.E.; Daak, S. Integration of phytochemicals and phytotherapy into cancer precision medicine. Oncotarget 2017, 8, 50284–50304.
- Jadhav, N.R.; Nadaf, S.J.; Lohar, D.A.; Ghagare, P.S.; Powar, T.A. Phytochemicals formulated as nanoparticles: Inventions, recent patents and future prospects. Recent Pat. Drug Deliv. Formul. 2017, 11, 173–186.
- Duan, T.; Xu, Z.; Sun, F.; Wang, Y.; Zhang, J.; Luo, C.; Wang, M. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed. Pharmacother. 2019, 117, 109121.
- Zhang, L.; Wu, C.; Mu, S.; Xue, W.; Ma, D. A chemotherapeutic self-sensibilized drug carrier delivering paclitaxel for the enhanced chemotherapy to human breast MDA-MB-231 cells. Colloids Surf. B Biointerfaces 2019, 181, 902–909.
- Monteiro, L.O.F.; Fernandes, R.S.; Castro, L.; Reis, D.; Cassali, G.D.; Evangelista, F.; Loures, C.; Sabino, A.P.; Cardoso, V.; Oliveira, M.C.; et al. Paclitaxel-loaded folate-coated pH-sensitive liposomes enhance cellular uptake and antitumor activity. Mol. Pharm. 2019, 16, 3477–3488.
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148.
- Baek, J.-S.; Cho, C.-W. A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells. Oncotarget 2017, 8, 30369–30382.
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1399–1406.
- Jabri, T.; Imran, M.; Aziz, A.; Rao, K.; Kawish, M.; Irfan, M.; Malik, M.I.; Simjee, S.U.; Arfan, M.; Shah, M.R. Design and synthesis of mixed micellar system for enhanced anticancer efficacy of Paclitaxel through its co-delivery with Naringin. Drug Dev. Ind. Pharm. 2019, 45, 703–714.
- Emami, J.; Rezazadeh, M.; Mashayekhi, M.; Rostami, M.; Jahanian-Najafabadi, A. A novel mixed polymeric micelle for co-delivery of paclitaxel and retinoic acid and overcoming multidrug resistance: Synthesis, characterization, cytotoxicity, and pharmacokinetic evaluation. Drug Dev. Ind. Pharm. 2018, 44, 729–740.





