
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lia Queiroz do Amaral | + 2160 word(s) | 2160 | 2021-07-21 09:45:02 | | | |
| 2 | Ron Wang | -210 word(s) | 1950 | 2021-08-03 04:02:02 | | |
Video Upload Options
Supramolecular Aggregates cross several disciplines, embracing the sciences of nature and joining theory, experiment, and application, from molecular to macroscopic levels. The problems of interdisciplinarity are overcome initially with scientific divulgation, bringing concepts from their origin, to facilitate the access of young scientists to the scientific content. Next, focus on some basic principles can help to understand the non trivial connections between Physics, Chemistry and Biology.
1. Introduction
Knowledge evolves in each discipline with its own specific criteria, usually without conceptual bridges among them. This article aims to help non trivial synthesis in the highly multidisciplinary field of Supramolecular Aggregates (SUMA).
In the introduction of a recently published paper [1] I have followed the evolution of studies of SUMA, initially in two different directions: colloid science and supra molecular chemistry, both starting still in the 19th century, as well as the evolution of Liquid Crystals (LC), both as thermotropics (dependent on temperature) and as lyotropics (dependent on relative composition). Polymers are known since antiquity, as well as soaps, and found uses before initial rationalization, in the 19th century. Changes occurred when the so called Exact Sciences evolved, with the integration of Chemistry with Physics, at the turn of the 19th to the 20th century. Modern Physics came after the definition of Chemistry as a true Science, with the construction of the periodic table of the elements. In the early 1900’s Physics found its way focusing on quantum mechanics and turning to the microscopic world below the hydrogen atom, while chemistry focused on molecules and above, towards the human world, and chemical industries helped to change human societies. Quantum chemistry dominated the scene in chemistry until the discovery of the structure of DNA, in the 1950’s. The second half of the 1900’s saw the emergence of biochemistry and molecular biology, a serious attempt of integration of the natural sciences. References on these subjects can be found in [1].
The discovery of biopolymers, natural polymers produced by the cells of living organisms opens the Pandora box of modern knowledge, as exists in the 21th century [2].
Here the focus is on some experimental results on problems which draw my attention and could be solved with specific theoretical approaches, based on simple physical concepts. I have selected one subject from [1] for this entry.
2.Theory VS. Experiment
The discussion on the relative importance of theory and experiment has no trivial answer, knowledge is constructed from both. Sometimes an experiment is planned to test a theory, but many times an experimental result is obtained for practical reasons, and the search for a theoretical explanation comes later on. This depends also on the field being considered, and on its historical development.
My own trajectory, starting as a rigorous student of Physics, good in mathematics, lead me to an initial 12 years experience in a research reactor, bound to the technique of slow neutron scattering, but moving from Nuclear Physics to Molecular Physics, including an experience abroad of 15 months in Stockholm, Sweden. This route, followed by academic degrees (Master in Nuclear Science and Technology and Ph.D. in Physics) was already discussed in [1], and was interrupted by maternity, I quitted that job.
Later on, it was possible for me to re-start from zero, with a provisional part time job at the Institute of Physics of USP, Brazil (IFUSP), giving classes of basic physics (theory and laboratories) for the two initial years of the Engineering courses. A proposal for research was then made, starting a new X-ray Crystallography laboratory (CrysLab). Looking for a new subject of research, I attended a seminar of a Canadian chemist that was visiting the NMR group of the Institute of Chemistry of USP. From this I could define a research project on lyotropic liquid crystals (in close connection with biomembranes). It should be a colaboration with the NMR group of the Chemistry Institute, where the samples (aqueous mixtures of water / detergent / additives) were prepared. The NMR research was focused on the structure of molecules (such as benzene) oriented by the lyotropic systems, while the IFUSP project proposed X-ray structural study of the lyotropic phases.
The expertise acquired with neutrons in research reactors was of little help, I needed to enter by myself on several aspects of Chemistry and Biology, some of them already discussed in [1]. Here the interest is to focus some specific theoretical approaches able to account for experimental results, which could not be understood from exact physical theories, but could advance applying correct physical concepts.
I have worked mainly at the interfaces physics / chemistry / biology / education, and at the interface nature sciences / human issues.
The discussion on the importance of applications of the acquired scientific knowledge is also in order, with some focus on the question basic vs applied science. The search for knowledge comes in humans from innate curiosity, since early childhood, from the wish / necessity to understand the world around each one of us, not necessarily from material needs. Therefore the drive for basic research is in general not the same as for applied research, and applications can develop only after basic knowledge has been acquired.
Basic research in nature sciences aims to understand the interactions in matter, while engineering focuses processes under control, for uses in human activities. My aim is to link basic knowledge with innovative ideas. Some specific results are discussed in the next item.
3.Self-assembly and lyotropic liquid crystals (LLC)
Research on lyotropic complex systems (water / amphiphile / additives) requires a very large amount of previous knowledge, from both Physics and Chemistry. Focus on experimental concepts is necessary, besides some knowledge on theories. A purely theoretical approach is still unable to deal with the very complex multicomponent aqueous solutions to be discussed now.
Starting from Physics, it is required a good background in the states of matter and their phase transitions, in concepts of order and disorder in structures of crystals and polycrystalline, in phase diagrams as a function not only of pressure and temperature (as for a single component), but also of relative concentration in multi component systems, as done in Metallurgy.
Starting from Chemistry, the focus must be in water and its anomalies, the structure of the water molecule and the effects of the H bond, ionization of water and pH, hydrophobic and hydrophilic effects, formation of micelle aggregates by self assembly, critical micelle concentration, behavior of soaps and detergents in water solution, phase diagrams as a function of relative concentration and temperature.
Figure 1 gives some idea about micelles, the transient aggregates formed in equilibrium with monomers in solution, changing forms with concentration and temperature. At the cmc single molecules form spherical micelles in the isotropic I phase, with increasing concentration Hexagonal Hα and Lamellar Lα phases may form, at temperatures above the Krafft line that separates these structures from gel and coagel crystalline phases. Complex cubic structures may form between Hα and Lα phases.
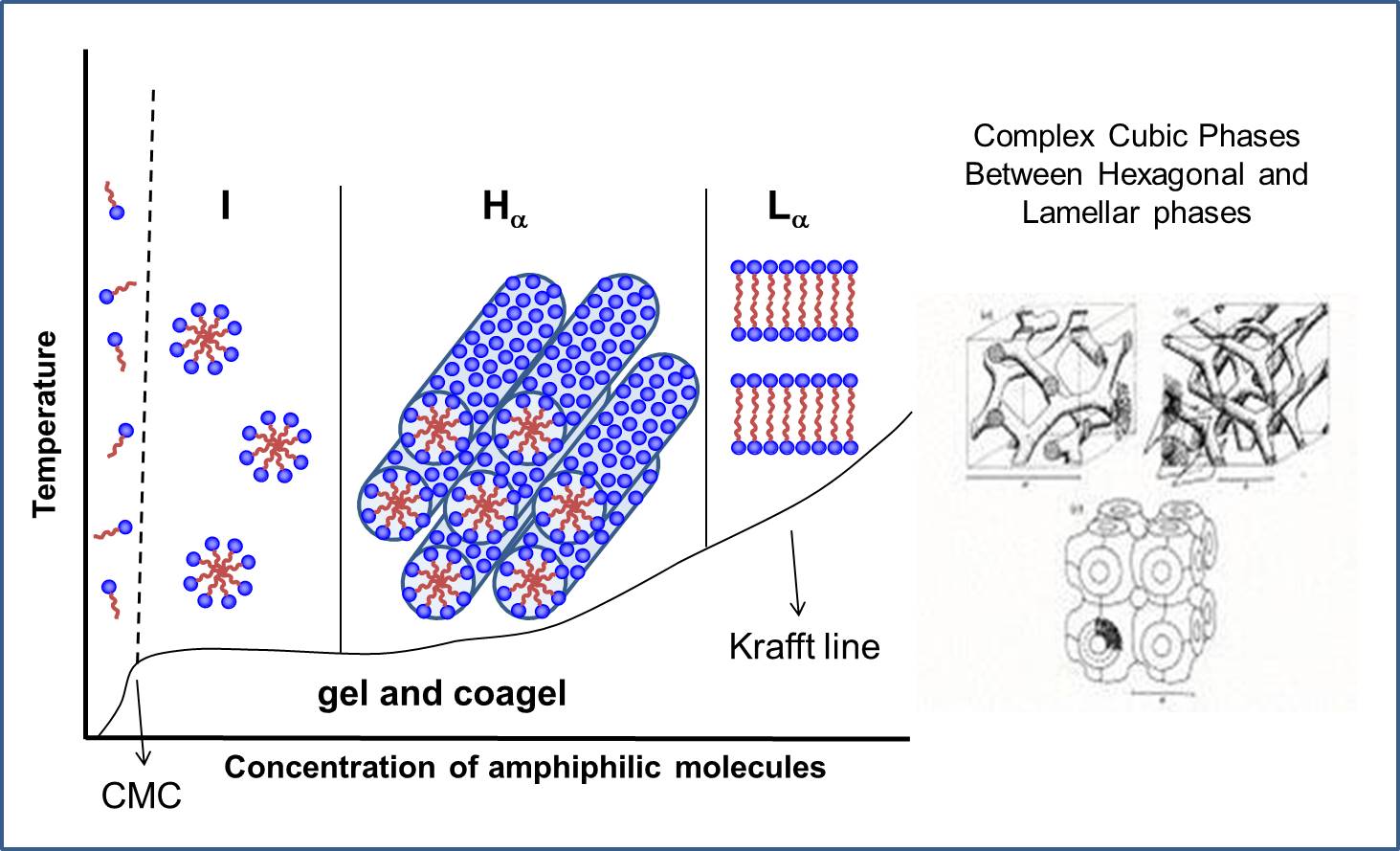
Figure 1 - Scheme of phase diagram as a function of concentration and temperature for a lyotropic aqueous system.
An important point to emphasize is that the hydrocarbon chains (HC) inside the aggregates in lyotropic liquid crystals (LLC) are in a disordered α state above the Kraft temperature, while they are in extended β conformation below that temperature. The state of the HC chains can be determined from X-ray diffraction, from the peak corresponding to average distance between the HC chains, diffuse at 4.5 Å in α state and sharp at 4.1 Å in β state.
In order to enter in this new field, I needed to study the whole problem of self-assembly, by that time unknown by physicists. The thermodynamics of micelle formation was being worked out by Tanford [3].
When the existence of the two uniaxial nematic phases was discovered by the chemist (prof. Reeves) with whom I interacted to propose the research project at IFUSP [4], they were classified as Type I and Type II, depending on their orientation on the magnetic field by NMR measurements on samples of detergent / deuterium / additives (decanol and /or salt), with positive and negative bulk diamagnetic anisotropy. A next paper was published [5] with a table summarizing all the phases known at that time, which already defined Type I as cylindrical (CM) and Type II as discotic (DM) micelles, and their relationship to the parent “hexagonal” and “lamellar” lyotropic liquid crystals. They also had different surface orientations, so that it was very clear that micelles changed symmetry with changes in composition.
This was five months before the paper on the discovery of the biaxial phase [6], intermediate between the two uniaxial phases in a specific ternary system (with potassium laurate), investigated by temperature variation in a specific sample composition, related to the entrance of physicists into this field.
Since the beginning, it was clear that there was a change in the symmetry of the micellar object, and that this could happen easily only in a lyotropic system, since thermotropic LC did not present a change in molecular symmetry.
Physicists were studying the complex ternary system with only two detergents, difficult to synthesize and buy, and fixing the weight composition.
In our lab we also started with such systems, produced by our chemist collaborators, working in the two uniaxial nematic phases. But I soon realized that the ternary system is too complex for physicists, and it became clear that the available theories were unable to explain the biaxial phase that is intermediate between the Nc uniaxial phases (cylindrical micelles) and the Nd uniaxial phases (discotic micelles).
So, instead of focusing on the elusive biaxial phase, I decided that it was necessary to discover the lyotropic nematic domain in another system of broader interest, and the choice was to focus on sodium dodecyl sulfate (SDS), also named sodium lauryl sulfate (SLS), chemical formula NaC12H25SO4, the standard amphiphile used for micellar systems in physical chemistry, which is easy to buy. It is a simple hydrocarbon chain (n = 12), with a terminal CH3 and a polar head, (SO3)- Na+. This allowed us to continue research in a more independent way.
With some effort, using polarized optical microscopy (POM) and surface effects in capillaries, it was possible to discover (by trial and error) an NC phase at 23°C with weight composition 25.00% SLS/ 70.53% H2O / 4.47% decanol [7].
A systematic search of the nematic domain with SLS was then made as a function of two concentration variables, the water / amphiphile (Mw) and the decanol / amphiphile (Md) relative molar ratios, with good results [8].
The ternary phase diagram at room temperature showed the nematic domain between the isotropic I, hexagonal Hα, and lamellar Lα phases already studied by chemists. The phase diagram as a function of temperature and Md indicated a change in micellar form at the transition Nd–Nc, for Md = 0.38, with Mw varying in the interval 40–45. Furthermore, a comparison with sodium decyl sulfate and K laurate in terms of the new variables Mw and Md (Table II of [8]) revealed that the transition between the two uniaxial forms occurred for very near Md values, while Mw had a large variation (from 21 to 43), depending on the size of the micellar aggregate. These original results defined new directions for the research, not only in our laboratory, but also in groups of chemists working on LLC, that did not enter before on the nematic LLC.
Instead of describing all the steps of the research done throughout more than three decades on the effect of decanol in the micellar structure (including 3 Ph.D. theses and many papers), it is more efficient to make reference to an invited paper published in the volume of Liquid Crystals in honor of Saupe, discussing the up-to-now unsolved problem of the micelles in the biaxial nematic phase [9], with 91 references.
Many other aspect of my research work in SUMA can be found in [1].
References
- Amaral, L.Q.d., Supramolecular Aggregates: Hardness Plus Softness from Journal of Molecules, 2021. 26(14). 4223.
- Folding, Assembly, and Persistence: The Essential Nature and Origins of Biopolymers Calvin M. Runnels,· Kathryn A. Lanier, Justin Krish Williams, Jessica C. Bowman, Anton S. Petrov, Nicholas V. Hud, Loren Dean Williams, Journal of Molecular Evolution (2018) 86:598–610, https://doi.org/10.1007/s00239-018-9876-2
- Tanford, C., Thermodynamics of micelle formation: Prediction of micelle size and size distribution chemistry. Proceedings of the National Academy of Science, 71(5), p. 1811-1815 (1974).
- Radley, K.; Reeves, L.W.; Tracey, A.S. Effect of Counterion Substitution on the Type and Nature of Nematic Lyotropic Phases from Nuclear Magnetic Resonance Studies. J. Phys. Chem. 1976, 80, 174–182.
- New Lyotropic Liquid Crystals Composed of Finite Nonsphericai Micelles, BRUCE J. FORREST and LEONARD W. REEVES, Chemical Reviews 81 (1) pp 1- 14, 1981.
- Yu, L.J.; Saupe, A. Observation of a Biaxial Nematic Phase in Potassium Laurate-1-Decanol-Water Mixtures. Phys. Rev. Lett. 1980, 45, 1000–1003.
- Amaral, L.Q.; Helene, M.E.M.; Bittencourt, D.R.; Itri, R. New nematic lyomesophase of sodium dodecyl sulfate. J. Phys. Chem. 1987, 91, 5949–5953.
- Amaral, L.Q.; Helene, M.E.M. Nematic domain in the SLS/H2O/decanol system. J. Phys. Chem. 1988, 92, 6094–6098.
- Amaral, L.Q. Micelles forming biaxial lyotropic nematic phases. Liq. Cryst. 2010, 37, 627–640.





