Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stéphane Bolduc | + 3356 word(s) | 3356 | 2021-07-23 04:15:47 | | | |
| 2 | Lily Guo | Meta information modification | 3356 | 2021-07-26 05:13:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bolduc, S. Genitourinary Tissue Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/12377 (accessed on 07 February 2026).
Bolduc S. Genitourinary Tissue Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/12377. Accessed February 07, 2026.
Bolduc, Stéphane. "Genitourinary Tissue Engineering" Encyclopedia, https://encyclopedia.pub/entry/12377 (accessed February 07, 2026).
Bolduc, S. (2021, July 23). Genitourinary Tissue Engineering. In Encyclopedia. https://encyclopedia.pub/entry/12377
Bolduc, Stéphane. "Genitourinary Tissue Engineering." Encyclopedia. Web. 23 July, 2021.
Copy Citation
Tissue engineering is an emerging field of research that initially aimed to produce 3D tissues to bypass the lack of adequate tissues for the repair or replacement of deficient organs.
tissue engineering
urethra
vagina
urology
gynaecology
epithelium
1. Introduction
Despite the significant advances in medicine of the last decades, many illnesses are still challenging. Indeed, the treatments themselves can be insufficient, or their side effects may negatively impact the patient’s quality of life. From congenital anomalies to various injuries or illnesses that may develop, the list goes on and can range from a simple annoyance to a deadly threat. Affectations of the genitourinary tissues are no exception in this respect and can significantly contribute to undermining the social and emotional life of individuals [1][2][3][4][5]. In this context, a particular focus will be placed on the male urethra and the vagina. This review examines the anatomy, pathologies and treatments. Current treatments will be studied, as well as encountered problems and possible solutions offered by tissue engineering. The field of tissue engineering has emerged in the past decades, aiming to treat or replace damaged tissues. Various approaches will be described, and some of their applications either repair the urethra and the vagina or create study models. A new and exceptionally innovative approach will then be put forward: The self-assembly technique [6].
2. Tissue Engineering
In 1993, Langer and Vacanti published a paper in Science entitled “Tissue Engineering” (TE) [7]. This was the first assay to conceptualise the notion of TE and its goals. From this date, TE has been considerably developed to offer new and innovative solutions to old problems. In the context of ageing and the sedentary behaviour of human populations, the appearance of chronic diseases that require organ repair or replacement is increasing even though the number of organs available for transplants is reducing precisely because of this ageing and sedentary behaviour, but also the strengthening of selection criteria by regulatory agencies. Tissue engineering consists of the in vitro reconstruction of tissues or organs for the replacement/repair and 3D models for fundamental research. Synthetic or natural biomaterials, including decellularised organs as scaffolds, seeded or not with cells, have been studied. Various techniques have been described to create the scaffold, such as casting [8], electrospinning [9][10][11] or bioprinting [12]. Other methods like the self-assembly technique, which does not require any exogenous biomaterial, have also been developed.
2.1. Synthetic Materials
Synthetic biomaterials present many advantages, such as their low cost, ability to be highly tunable and form the desired geometry [13][14][15]. They also present some drawbacks, such as their difference in composition and organisation compared to a natural extracellular matrix (ECM), which can impact adhesion, proliferation and differentiation of cells [16]. Indeed, the low diversity of the components used to produce these biomaterials appears simplistic compare to the numerous components of a native ECM. Nevertheless, significant progress has been made with the functionalisation of synthetic biomaterials in the last decades. For many applications, the use of hydrogels is interesting, and recent studies have shown a potential for them functionalised with different peptides to recreate, at least in part, the microenvironment of the target tissues [17]. This kind of functionalised hydrogels could support cell growth/differentiation, as well as vehicles for the delivery of stem cells, drugs or bioactive proteins.
2.2. Natural Materials
Contrarily to synthetic materials, natural biomaterials used as scaffolds, especially those using elements coming from ECM [18], provide better adapted environments for migration, proliferation and differentiation of cells when used under appropriate conditions. Nevertheless, these biomaterials generally have weaker mechanical properties compared to native ECM. Indeed, the mechanical properties of ECM are not only due to the molecules it is composed of, but also, more importantly, because the organisation of these molecules in the matrix.
Among other promising biomaterials, decellularised tissues are becoming more and more attractive with new protocols of decellularisation [19]. Indeed, it seems convenient to use the complexity of native materials to repair themselves. Decellularising tissues to remove antigenic molecules responsible for immunological response and potential pathogens before being recellularised with patient’s cells allows tissue production, which is very close to the autograft or even better if the disease’s causal effect is also corrected. Decellularisation of tissue can be obtained by successive hypo/hypertonic shocks, combined or not with the addition of detergents, to destroy the cell membrane [20]. Enzymes can be used to remove proteins and nucleic acids. The tissue can originate from a human cadaver or be obtained from animals.
Nevertheless, several problems slow down the use of such prostheses: Ethical problems and various regulatory issues, such as the risk of immune reaction in ineffective/incomplete decellularisation. However, the main problem remains the ability to maintain the ECM architecture that provides good mechanical and biological characteristics to the graft. Indeed, heavy decellularisation can induce a loss of the native matrix architecture and its non-immunogen components, whereas weak decellularisation could be insufficient to obtain a safe biomaterial [21][22][23]. In the past, ECM was largely disorganised or modified by the decellularisation process, losing the mechanical properties, resistance, elasticity and cell differentiation potential. An increasing number of protocols are emerging to reduce these problems, but more studies are needed to obtain a clear view for this option [24]. Recently a novel approach to functionalise decellularised tissues has been proposed to improve endothelial cell adhesion and accelerates endothelialisation, which is an important point, due to the need to rapidly provide oxygen and nutrients to grafts to ensure positive outcomes. This goal is achieved through selective immobilisation of REDV tetrapeptides [25].
2.3. Tissue Engineering for Urethral Reconstruction
Several groups have attempted urethral substitution using TE cell-free matrices, such as bladder acellular matrix graft (BAMG) and small intestinal submucosa (SIS) or cellularised matrices [26][27][28][29][30][31][32][33]. These matrices are prepared from native tissues by decellularising and sterilising them. As shown in rabbits by Dorin et al., a significant problem of acellular matrices is that urothelial regeneration is limited to 0.5 cm, which compromises success in more complex cases, such as long strictures [34]. Synthetic polymers have also shown advantages (poly-l-lactic acid, (PLLA) and poly(lactic-co-glycolic) acid, (PLGA)) for forming low-cost, biocompatible, three-dimensional (3D) organs with controlled mechanical properties. However, synthetic scaffolds without functionalisation by peptides do not allow the proper differentiation of epithelial cells into well-organised tissue. Indeed, contrarily to natural matrices, they cannot recreate the target organ microenvironment, especially adequate ECM-cell interaction (e.g., lack integrin-binding peptide sequence, failure in synchronisation between degradation rate and matrix neo-deposition) [13][35][36]. No long-term experiment has been performed with a significant number of patients. Currently, protocols developed are not used in clinics despite the media coverage of some, signalling the immaturity of the works, which must continue to be improved [37]. TE substitutes that contain autologous cells in addition to an extracellular matrix, close to the native one, are more promising. The main advantage of this method is that a large graft of autologous cells can be produced with a limited sample, such as a piece of oral or bladder mucosa. Indeed, the extracted cells can be grown in vivo, seeded on the biomaterial and implanted with a very low risk of rejection. Studies have also reported that stem cells can be obtained from urine, making this approach potentially useful [38][39]. A downfall of this method is that after long periods of culture to obtain well-differentiated tissues, the exogenous matrices become challenging to manipulate and lose their mechanical and physical properties. Despite significant progress in urethral TE, very few teams have performed clinical trials and published their results to date [40] (Table 1). However, the four clinical trials conducted to date show promising results in a limited number of patients with long-segment and/or complex stenosis [37][41][42][43][44]. While these models are certainly far from a “plug and play” alternative with consistently reproducible results, they could offer an alternative for complex cases requiring long segment urethral replacement [45].
Table 1. The scaffold used for urethral reconstruction.
| Type of Scaffold | Biomaterials | Ref Example | Advantages | Drawbacks |
|---|---|---|---|---|
| Synthetic | PLCL | [46] | - biocompatible - mechanical properties |
- Degradation products |
| PLCL/Collagen | [47] | - low cost | - Poor differentiation of epithelial cells (except for cellularised collagen matrices; improved by functionalisation) | |
| PLA | [48] | - highly reproducible | -degradation rate (too low or too high) | |
| PU/mesh in PGA | [49] | - quickly available | -mechanical properties during or after degradation | |
| PLGA | [37] | - functionalisation | - poor angiogenesis | |
| PLLA | [50] | |||
| Natural | Cellulose | [51] | ||
| Silk Fibroin | [26][52][53][54] | |||
| Collagen | [18][28][55][56][57][58][59] | |||
| Acellular matrix | SIS | [21][60][61][62][63][64][65] | - Adequate microenvironment for cell proliferation and differentiation | - Immune risk (including DNA, prions) |
| Placental membrane | [66] | - Significant angiogenesis | - Unfavourable clinical experience | |
| BAMG | [67][68][69] | - Quality of the matrix | ||
| Urethra | [70] | |||
| Self-Assembly | None | [13][71][72] | - Excellent microenvironment with organ-specific cells - Mechanical properties |
- time and cost to produce tissues |
2.4. Tissue Engineering for Vaginal Reconstruction
Vaginal abnormalities represent a significant health problem for women because nearly 1% of women will suffer from the pathologies mentioned previously, resulting in significant psychological impacts. Interestingly, tissue engineering is an area that aims to replace or regenerate dysfunctional tissues and organs with autologous cells, biomaterials or a combination of both. The success of vaginal reconstruction in these patients largely depends on the use of a sufficiently large tissue substrate that adequately fulfils the physiological functions of the vagina. Prior techniques have often relied on autologous tissues, such as the intestine or skin, often associated with complications, due to the physiological differences inherent to these substrates. To improve results, a variety of biodegradable substitutes, including collagen matrices and a decellularised bladder submucosa, have been used for vaginal replacement [42][73]. Reconstructions using these substitutes have generally failed, due to functional, structural, mechanical or biocompatibility issues. Using the patient’s vaginal tissue for reconstruction would be the most elegant and effective solution, but this has often not been possible, due to the relative scarcity of healthy vaginal tissue for autologous transplantation. There is a substantial clinical need to develop technologies to facilitate the regeneration of injured or diseased tissues and organs. The relentless prevalence of trauma, congenital abnormalities and diseases, such as cancer, is driving demand, becoming increasingly urgent as the world’s population grows and ages. A wide variety of tissues and organs would benefit from engineering-based repairing or regeneration. Several graft materials have been used to line the surgically created neovaginal cavity, including myocutaneous flaps or intestinal segments, full-thickness or split-thickness skin grafts, amniotic membrane, peritoneum, buccal mucosa and vaginal epithelial tissue [74][75][76][77][78][79][80][81][82]. These techniques are associated with contraction and/or stenosis of the graft, which may require long-term dilation. Oral mucosa vaginoplasties are associated with donor site morbidities, due to the large volume of tissue taken to create the neovagina. In addition, the amount of tissue that can be harvested from a donor site is limited, which can be problematic, especially for significant defects. To overcome these difficulties, alternative methods of vaginal reconstruction have been explored. Few groups have attempted TE vaginal reconstruction using acellular and cellular matrices of natural or synthetic origin [83][84][85][86][87][88] (Table 2). The tissues were transplanted into mice, rabbits and humans. However, further preclinical and clinical studies are needed, due to the limited number of subjects included in these studies. It remains challenging to determine whether the optimal technique was used.
Table 2. The scaffold used for vaginal reconstruction.
| Type of Scaffolds | Biomaterials | Patients # | References |
|---|---|---|---|
| Synthetic | PGA | 4 | [88] |
| PLA (©PACIENA) | 9 | [89] | |
| 7 | [90] | ||
| Natural | Collagen IV and hyaluronic acid | 1 | [85] |
| 23 | [85] | ||
| Acellular matrix | Amnion | 50 | [91] |
| SIS | 65 (vs Interceed) | [92] | |
| Monkey | [93] | ||
| Acellular vaginal matrix | Rat | [94] | |
| Rat | [87] | ||
| Artificial dermis | 35 | [95] | |
| Self-Assembly | Mouse | [96][97] |
3. The Self-Assembly Approach
New approaches are required to combine adequate cell signalling and differentiation, cell maintenance, especially for stem cells, and sufficient mechanical resistance for implantation. All this while minimising side effects. A new type of strategy has been explored by the group of Dr. François A. Auger at LOEX: The “self-assembly” method [6]. Great discoveries and therapeutic achievements have been made possible thanks to this unique technique which allows the production of reconstructed tissues free of exogenous materials. Indeed, the use of exogenous biomaterials can lead to immunological, foreign body reactions and the transmission of infections. This technique relies on cells cultured in ascorbic acid to secrete and deposit their own ECM to form cohesive sheets of cells and collagen [98][99]. While most biomaterials lose their mechanical and physical strength properties in culture, these self-assembled tissue properties are roughly similar or even exceeding those of native tissues in some models, due to the stabilisation of metalloproteinases [100]. The self-assembly technique has made it possible to construct models from various stromal cells of the skin [101], fat [102], cornea [103], Warton’s jelly [104], bladder [105] and vagina [96] exhibit not only excellent mechanical strength, but also an allowance for adequate epithelial differentiation.
The first step of a tissue’s reconstruction using the self-assembly technique is to seed mesenchymal cells and cultivate them in the presence of ascorbate, also called vitamin C. It is preferable to use organ-specific mesenchymal cells (e.g., dermal fibroblasts to reconstruct skin substitutes, keratocytes to reconstruct cornea substitutes [106], bladder mesenchymal cells to reconstruct bladder mucosa substitutes [107] and vagina fibroblasts to reconstruct vaginal mucosa substitutes [108]). The use of unpaired mesenchymal cells can result in inadequate differentiation. Indeed, cutaneous differentiation of corneal or urothelial epithelial cells occurs when these cells are cultivated on dermal fibroblasts-derived stromas. The ascorbate concentration was set at 50 µg/mL even if it is not the optimal concentration for collagen deposition in dermal fibroblast cell culture. Indeed, higher concentrations of this oxidative agent induce cell death, and thus, reduce the total amount of deposited collagen and the mechanical strength of the final product. After 3 to 6 weeks, depending on the ability of the cultivated cells to deposit ECM, the mesenchymal cells have produced a thick material similar to a native stroma of the target organ (Figure 1). It is possible to refine the protocol by introducing, for example, at the initial seeding step, endothelial cells from an umbilical vein or, even better, from the organ-specific microvascular network to form a capillary-like network [109] or immune cells, such as monocyte-derived macrophages to produce immunocompetent models [108]. A reseeding step can also be done after two weeks to increase the tissue thickness and improve the cell distribution inside the stroma, which can especially be helpful for the microvascular network and increase elastic properties of the models [109] (Figure 2A). Following the step of stroma production, epithelial cells can be seeded on the top of the construct and cultivated for one week in submerged conditions, allowing the complete coverage of its upper surface before being placed at the air/liquid interface for three weeks to obtain differentiation of the epithelium. This technique has shown a high level of differentiation in various models, showing epitheliums very similar to native tissues. To place bladder tissues in physiological conditions, a bioreactor has been designed (Figure 2B). Detailed protocols can be found in several research articles and reviews [110].
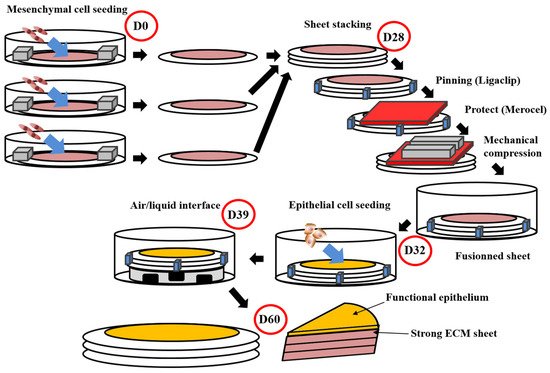
Figure 1. Schema of the self-assembly method using the standard protocol. This protocol obtains flat tissues with functional epithelium and a strong ECM sustaining manipulations by surgeons or researchers [105][111][112]. The total time of production around 60 days. For the whole process, media are supplemented with ascorbate (50 µg/mL). On day 0 (D0), mesenchymal cells are seeded into petri dishes containing a paper ring as an anchorage device weighted by small metal lingots. Cells are then cultured for 28 days. On day 28 (D28), stroma sheet (cells + ECM) are stacked (air bubble must be avoided between sheets), then they are pinned together using a surgical clip (Ligaclip), covered by a surgical sponge (Merocel) to protect from the direct contact with metal lingots use to favour fusion through mechanical compression. Culture is pursued for 4 days until day 32 (D32) to ensure adequate fusion. Then epithelial cells are seeded on the top of the construct and cultured for a week to allow horizontal coverage of the scaffold. On day 39 (D39), the constructs are raised at the air/liquid interface using a specific device allowing media circulation under the reconstructed tissue. This phase allows the maturation of epithelium until day 60 (D60).
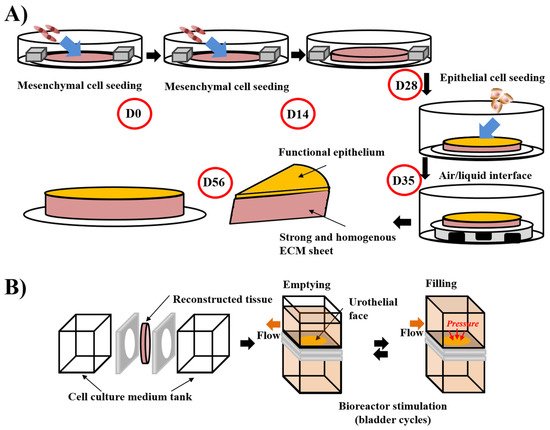
Figure 2. Schema of the “reseeding” variation of the self-assembly protocol and the stimulation of bladder mucosa substitute in a bioreactor. (A) The “reseeding” protocol reduces the cost and the complexity of the self-assembly technique compared to the standard protocol, avoiding the need for multiple sheets and their stacking. A better distribution of the cells throughout the tissue has also been demonstrated. (B) Stimulation of bladder mucosa substitute using bioreactor. The substitute is inserted between two chambers where the cell culture medium can circulate. To mimic the bladder cycles of emptying and filling, the flow is modified and increased the pressure on the urothelial face of the substitute in the filled status, whereas the substitute is more relaxed in the empty condition.
For tubular substitutes, such as blood vessels or urethras, the epithelial/endothelial seeding does not happen immediately after the step of stroma production. The tissue-like structure is detached from the petri dish and tightly rolled around a mandrel of the appropriate diameter [100]. After the rolling step, the fusion of the rolls was helped by maintaining a mechanical load. The cylindrical mandrel can then be removed from the tubular structure, creating a lumen. The lumen is filled with liquid to avoid collapse, and epithelial/endothelial cells can be seeded inside the tube for urethral or blood vessel reconstruction, respectively. During the epithelial/endothelial cell seeding step, rotation of the tube ensures a uniform distribution of these cells (Figure 3A). Once again, urologic tissues can be matured under physiological conditions using bioreactors (Figure 3B). Various refinement has been introduced for these constructs. Notably, mesenchymal cells can be seeded directly on the mandrel to form the tubular structure, avoiding delamination of the rolls in the case of their incomplete fusion [113].
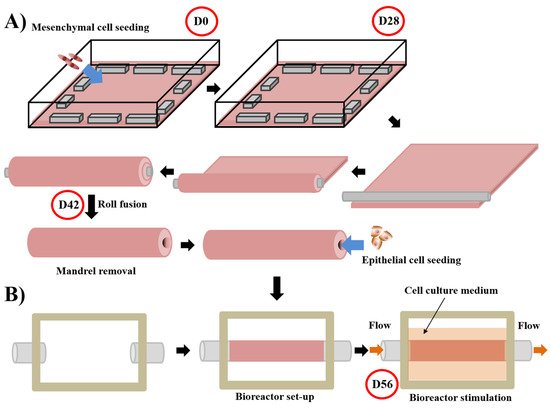
Figure 3. Schema of the technique used to produce tubular substitute using the self-assembly approach and the subsequent stimulation in a bioreactor of the substitutes. For the whole process, media are supplemented with ascorbate (50 µg/mL). (A) Mesenchymal cells are seeded at day 0 (D0) on a gelatin-coated square plate and cultured for 28 days. Contraction of the tissue is avoided by putting flat metal lingots on its border. After the mesenchymal cells formed an ECM sheet (day 28 (D28)), the latter was rolled tightly around a cylindrical mandrel. Fusion of the rolls is allowed by maintaining a little load on the tissue for 14 days. On day 42 (D42), the mandrel can be removed and epithelial cells seeded inside the tubular structure [114][115]. (B) The day after, a bioreactor separates the external and internal parts of the tissue, a flow circulating in the internal part mimicking the physiological flow. The cell culture medium is present in the external part to provide nutrients. Maturation is done for 14 days until day 56 (D56) [116].
4. Perspectives
The field of tissue engineering can bring potential alternatives to the constantly increasing need for tissue repair/replacement of urological organs. To do so, a real challenge is to combine good mechanical properties while maintaining stem cell potential. It must also allow adequate epithelium differentiation, and its degradation product should not imply adverse effects. It must be vascularised easily and not represent an immune risk for patients, while being functional as soon as the graft is in place. We can, therefore, better understand the significant obstacle that must be overcome.
In this article, we detailed the self-assembly technique, without using synthetic or decellularised biomaterials/scaffolds. Indeed, genitourinary tissues have been developed using animal cells and have been successfully implanted in animals. To facilitate clinical translation and avoid interspecies differences, the next step is to graft human organ-specific tissues in immunosuppressed animals before trying the prototype on human subjects. These tissues constitute a promising avenue for the surgical correction of various defects, whether from congenital or acquired origin.
Several new challenges emerge, such as urothelial, mesenchymal and endothelial cells differentiated from iPSC derived from blood cells. This would avoid the need for a biopsy with its potential comorbidities, and avoid the problem of the non-organ-specific communications between cells leading to aberration or the presence of inadequate cells to harvest (e.g., cancer). However, there remains a lot to be done to obtain an adequate differentiation from iPSC without aberrant expression of oncogenes. Another challenge in the coming years should be to exclude the use of serum in cell culture. Serum could induce a loss of reproducibility in some experiments and be a source of contamination and raise ethical concerns. Furthermore, the world’s increase in demand will keep dragging the price up while the global production of serum is stagnant.
References
- Tyson, M.D., 2nd; Barocas, D.A. Quality of life after radical cystectomy. Urol. Clin. N. Am. 2018, 45, 249–256.
- Ahmadi, H.; Lee, C.T. Health-related quality of life with urinary diversion. Curr. Opin. Urol. 2015, 25, 562–569.
- Castro, R.A.; Arruda, R.M.; Bortolini, M.A. Female urinary incontinence: Effective treatment strategies. Climacteric 2015, 18, 135–141.
- Ofman, U.S. Preservation of function in genitourinary cancers: Psychosexual and psychosocial issues. Cancer Investig. 1995, 13, 125–131.
- Orvis, B.R.; Lue, T.F. New therapy for impotence. Urol. Clin. N. Am. 1987, 14, 569–581.
- Saba, I.; Jakubowska, W.; Bolduc, S.; Chabaud, S. Engineering tissues without the use of a synthetic scaffold: A twenty-year history of the self-assembly method. Biomed. Res. Int. 2018, 2018, 5684679.
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926.
- Boys, A.J.; Barron, S.L.; Tilev, D.; Owens, R.M. Building scaffolds for tubular tissue engineering. Front. Bioeng. Biotechnol. 2020, 8, 589960.
- Ercolani, E.; Del Gaudio, C.; Bianco, A. Vascular tissue engineering of small-diameter blood vessels: Reviewing the electrospinning approach. J. Tissue Eng. Regen. Med. 2015, 9, 861–888.
- Haghjooy Javanmard, S.; Anari, J.; Zargar Kharazi, A.; Vatankhah, E. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 2016, 31, 438–449.
- Muerza-Cascante, M.L.; Haylock, D.; Hutmacher, D.W.; Dalton, P.D. Melt electrospinning and its technologization in tissue engineering. Tissue Eng. Part B Rev. 2015, 21, 187–202.
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164.
- Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From acellular matrices to smart polymers: Degradable scaffolds that are transforming the shape of urethral tissue engineering. Int. J. Mol. Sci. 2019, 20, 1763.
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell Mol. Med. 2012, 16, 2247–2270.
- Jeong, S.I.; Kim, B.S.; Lee, Y.M.; Ihn, K.J.; Kim, S.H.; Kim, Y.H. Morphology of elastic poly(L-lactide-co-epsilon-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaffolds. Biomacromolecules 2004, 5, 1303–1309.
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308.
- Zamuner, A.; Cavo, M.; Scaglione, S.; Messina, G.M.L.; Russo, T.; Gloria, A.; Marletta, G.; Dettin, M. Design of decorated self-assembling peptide hydrogels as architecture for mesenchymal stem cells. Materials 2016, 9, 727.
- Tachibana, M.; Nagamatsu, G.R.; Addonizio, J.C. Ureteral replacement using collagen sponge tube grafts. J. Urol. 1985, 133, 866–869.
- Lin, C.H.; Hsia, K.; Ma, H.; Lee, H.; Lu, J.H. In vivo performance of decellularized vascular grafts: A review article. Int. J. Mol. Sci. 2018, 19, 2101.
- Guruswamy Damodaran, R.; Vermette, P. Tissue and organ decellularization in regenerative medicine. Biotechnol. Prog. 2018, 34, 1494–1505.
- Zhang, Y.; Frimberger, D.; Cheng, E.Y.; Lin, H.K.; Kropp, B.P. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006, 98, 1100–1105.
- Davis, N.F.; Cunnane, E.M.; O’Brien, F.J.; Mulvihill, J.J.; Walsh, M.T. Tissue engineered extracellular matrices (ECMs) in urology: Evolution and future directions. Surgeon 2018, 16, 55–65.
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80.
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 2020, 8, 10023–10049.
- Dal Sasso, E.; Zamuner, A.; Filippi, A.; Romanato, F.; Palmosi, T.; Vedovelli, L.; Gregori, D.; Gomez Ribelles, J.L.; Russo, T.; Gloria, A.; et al. Covalent functionalization of decellularized tissues accelerates endothelialization. Bioact. Mater. 2021, 6, 3851–3864.
- Chung, Y.G.; Tu, D.; Franck, D.; Gil, E.S.; Algarrahi, K.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R., Jr.; Mauney, J.R. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS ONE 2014, 9, e91592.
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015, 69, 45–55.
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217.
- Atala, A.; Danilevskiy, M.; Lyundup, A.; Glybochko, P.; Butnaru, D.; Vinarov, A.; Yoo, J.J. The potential role of tissue-engineered urethral substitution: Clinical and preclinical studies. J. Tissue Eng. Regen. Med. 2017, 11, 3–19.
- Chun, S.Y.; Kim, B.S.; Kwon, S.Y.; Park, S.I.; Song, P.H.; Yoo, E.S.; Kim, B.W.; Kwon, T.G.; Kim, H.T. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J. Korean Med. Sci. 2015, 30, 301–307.
- Li, C.; Xu, Y.M.; Liu, Z.S.; Li, H.B. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology 2013, 81, 1075–1080.
- Sartoneva, R.; Haaparanta, A.M.; Lahdes-Vasama, T.; Mannerstrom, B.; Kellomaki, M.; Salomaki, M.; Sandor, G.; Seppanen, R.; Miettinen, S.; Haimi, S. Characterizing and optimizing poly-L-lactide-co-epsilon-caprolactone membranes for urothelial tissue engineering. J. R. Soc. Interface 2012, 9, 3444–3454.
- Sun, D.; Yang, Y.; Wei, Z.; Xu, Y.; Zhang, X.; Hong, B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J. Cell Mol. Med. 2014, 18, 434–443.
- Dorin, R.P.; Pohl, H.G.; De Filippo, R.E.; Yoo, J.J.; Atala, A. Tubularized urethral replacement with unseeded matrices: What is the maximum distance for normal tissue regeneration? World J. Urol. 2008, 26, 323–326.
- Orlando, G.; Soker, S.; Stratta, R.J. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann. Surg. 2013, 258, 221–232.
- Jerman, U.D.; Kreft, M.E.; Veranic, P. Epithelial-mesenchymal interactions in urinary bladder and small intestine and how to apply them in tissue engineering. Tissue Eng. Part B Rev. 2015, 21, 521–530.
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182.
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233.
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132.
- Versteegden, L.R.M.; de Jonge, P.; IntHout, J.; van Kuppevelt, T.H.; Oosterwijk, E.; Feitz, W.F.J.; de Vries, R.B.M.; Daamen, W.F. Tissue engineering of the urethra: A systematic review and meta-analysis of preclinical and clinical studies. Eur. Urol. 2017, 72, 594–606.
- Engel, O.; Ram-Liebig, G.; Reiß, P.; Schwaiger, B.; Pfalzgraf, D.; Dahlem, R.; Fisch, M. 15 Tissue—Engineered buccal mucosa urethroplasty. Outcome of our first 10 patients. J. Urol. 2012, 187, e6.
- Fossum, M.; Skikuniene, J.; Orrego, A.; Nordenskjold, A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr. 2012, 101, 755–760.
- Bhargava, S.; Patterson, J.M.; Inman, R.D.; MacNeil, S.; Chapple, C.R. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur. Urol. 2008, 53, 1263–1269.
- Osman, N.I.; Patterson, J.M.; MacNeil, S.; Chapple, C.R. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur. Urol. 2014, 66, 790–791.
- Ramsay, S.; Ringuette-Goulet, C.; Langlois, A.; Bolduc, S. Clinical challenges in tissue-engineered urethral reconstruction. Transl. Androl. Urol. 2016, 5, 267–270.
- Sartoneva, R.; Nordback, P.H.; Haimi, S.; Grijpma, D.W.; Lehto, K.; Rooney, N.; Seppanen-Kaijansinkko, R.; Miettinen, S.; Lahdes-Vasama, T. Comparison of poly(l-lactide-co-varepsilon-caprolactone) and poly(trimethylene carbonate) membranes for urethral regeneration: An in vitro and in vivo study. Tissue Eng. Part A 2018, 24, 117–127.
- Kanatani, I.; Kanematsu, A.; Inatsugu, Y.; Imamura, M.; Negoro, H.; Ito, N.; Yamamoto, S.; Tabata, Y.; Ikada, Y.; Ogawa, O. Fabrication of an optimal urethral graft using collagen-sponge tubes reinforced with Copoly(L-lactide/epsilon-caprolactone) fabric. Tissue Eng. 2007, 13, 2933–2940.
- Wang, D.J.; Li, M.Y.; Huang, W.T.; Lu, M.H.; Hu, C.; Li, K.; Qiu, J.G.; Gao, X. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect. Tissue Res. 2015, 56, 434–439.
- Rapoport, H.S.; Fish, J.; Basu, J.; Campbell, J.; Genheimer, C.; Payne, R.; Jain, D. Construction of a tubular scaffold that mimics J-shaped stress/strain mechanics using an innovative electrospinning technique. Tissue Eng. Part C Methods 2012, 18, 567–574.
- Lv, X.; Guo, Q.; Han, F.; Chen, C.; Ling, C.; Chen, W.; Li, B. Electrospun poly(l-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int. J. Mol. Sci. 2016, 17, 1262.
- Huang, J.W.; Lv, X.G.; Li, Z.; Song, L.J.; Feng, C.; Xie, M.K.; Li, C.; Li, H.B.; Wang, J.H.; Zhu, W.D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005.
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110.
- Xie, M.; Xu, Y.; Song, L.; Wang, J.; Lv, X.; Zhang, Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J. Surg. Res. 2014, 188, 1–7.
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781.
- Vardar, E.; Engelhardt, E.M.; Larsson, H.M.; Mouloungui, E.; Pinnagoda, K.; Hubbell, J.A.; Frey, P. Tubular compressed collagen scaffolds for ureteral tissue engineering in a flow bioreactor system. Tissue Eng. Part A 2015, 21, 2334–2345.
- Sayeg, K.; Freitas-Filho, L.G.; Waitzberg, A.F.; Arias, V.E.; Laks, M.; Egydio, F.M.; Oliveira, A.S. Integration of collagen matrices into the urethra when implanted as onlay graft. Int. Braz. J. Urol. 2013, 39, 414–423.
- Larsson, H.M.; Vythilingam, G.; Pinnagoda, K.; Vardar, E.; Engelhardt, E.M.; Sothilingam, S.; Thambidorai, R.C.; Kamarul, T.; Hubbell, J.A.; Frey, P. Fiber density of collagen grafts impacts rabbit urethral regeneration. Sci. Rep. 2018, 8, 10057.
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Busch, S.; Daum, L.; Stenzl, A.; Amend, B.; et al. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: First results in a xenograft minipig model. World J. Urol. 2017, 35, 1125–1132.
- Micol, L.A.; Arenas da Silva, L.F.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.; Frey, P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455.
- Guo, H.; Sa, Y.; Huang, J.; Wang, Z.; Wang, L.; Xie, M.; Lv, X. Urethral reconstruction with small intestinal submucosa seeded with oral keratinocytes and TIMP-1 siRNA transfected fibroblasts in a rabbit model. Urol. Int. 2016, 96, 223–230.
- Orabi, H.; Safwat, A.S.; Shahat, A.; Hammouda, H.M. The use of small intestinal submucosa graft for hypospadias repair: Pilot study. Arab. J. Urol. 2013, 11, 415–420.
- Feil, G.; Christ-Adler, M.; Maurer, S.; Corvin, S.; Rennekampff, H.O.; Krug, J.; Hennenlotter, J.; Kuehs, U.; Stenzl, A.; Sievert, K.D. Investigations of urothelial cells seeded on commercially available small intestine submucosa. Eur. Urol. 2006, 50, 1330–1337.
- Zhang, Y.; Kropp, B.P.; Moore, P.; Cowan, R.; Furness, P.D., 3rd; Kolligian, M.E.; Frey, P.; Cheng, E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J. Urol. 2000, 164, 928–934; discussion 925–934.
- Fiala, R.; Vidlar, A.; Vrtal, R.; Belej, K.; Student, V. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur. Urol. 2007, 51, 1702–1708; discussion 1708.
- Palminteri, E.; Berdondini, E.; Colombo, F.; Austoni, E. Small intestinal submucosa (SIS) graft urethroplasty: Short-term results. Eur. Urol. 2007, 51, 1695–1701; discussion 1701.
- Pusateri, C.R.; Doudt, A.D.; Gauerke, S.; McCammon, K.; Qin, X.; Ork, B.; Khoury, J.M.; May, A.D.; Zuckerman, J.M. Placental membrane grafts for urethral replacement in a rabbit model: A pilot study. World J. Urol. 2020, 38, 2133–2138.
- Huang, J.W.; Xie, M.K.; Zhang, Y.; Wei, G.J.; Li, X.; Li, H.B.; Wang, J.H.; Zhu, W.D.; Li, C.; Xu, Y.M.; et al. Reconstruction of penile urethra with the 3-dimensional porous bladder acellular matrix in a rabbit model. Urology 2014, 84, 1499–1505.
- Li, H.; Xu, Y.; Xie, H.; Li, C.; Song, L.; Feng, C.; Zhang, Q.; Xie, M.; Wang, Y.; Lv, X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: An animal model. Tissue Eng. Part A 2014, 20, 774–784.
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: A preclinical study. Eur. Urol. 2013, 63, 531–538.
- Simoes, I.N.; Vale, P.; Soker, S.; Atala, A.; Keller, D.; Noiva, R.; Carvalho, S.; Peleteiro, C.; Cabral, J.M.; Eberli, D.; et al. Acellular urethra bioscaffold: Decellularization of whole urethras for tissue engineering applications. Sci. Rep. 2017, 7, 41934.
- Caneparo, C.; Chabaud, S.; Bolduc, S. Reconstruction of vascular and urologic tubular grafts by tissue engineering. Processes 2021, 9, 513.
- Chapple, C. Tissue engineering of the urethra: Where are we in 2019? World J. Urol. 2020, 38, 2101–2105.
- Wefer, J.; Sekido, N.; Sievert, K.D.; Schlote, N.; Nunes, L.; Dahiya, R.; Jonas, U.; Tanagho, E.A. Homologous acellular matrix graft for vaginal repair in rats: A pilot study for a new reconstructive approach. World J. Urol. 2002, 20, 260–263.
- Wu, S.; Cheng, Z.; Liu, G.; Zhao, X.; Zhong, L.; Zhu, Y.; Zhu, J. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal. Cell Pathol. 2013, 36, 63–69.
- Morton, K.E.; Davies, D.; Dewhurst, J. The use of the fasciocutaneous flap in vaginal reconstruction. Br. J. Obstet. Gynaecol. 1986, 93, 970–973.
- Hendren, W.H.; Atala, A. Use of bowel for vaginal reconstruction. J. Urol. 1994, 152, 752–755; discussion 756–757.
- Wiser, W.L.; Bates, G.W. Management of agenesis of the vagina. Surg. Gynecol. Obstet. 1984, 159, 108–112.
- Morton, K.E.; Dewhurst, C.J. Human amnion in the treatment of vaginal malformations. Br. J. Obstet. Gynaecol. 1986, 93, 50–54.
- Zhou, J.H.; Sun, J.; Yang, C.B.; Xie, Z.W.; Shao, W.Q.; Jin, H.M. Long-term outcomes of transvestibular vaginoplasty with pelvic peritoneum in 182 patients with Rokitansky’s syndrome. Fertil. Steril. 2010, 94, 2281–2285.
- Ding, J.X.; Zhang, X.Y.; Chen, L.M.; Hua, K.Q. Vaginoplasty using acellular porcine small intestinal submucosa graft in two patients with Meyer-von-Rokitansky-Kuster-Hauser syndrome: A prospective new technique for vaginal reconstruction. Gynecol. Obstet. Investig. 2013, 75, 93–96.
- Lin, W.C.; Chang, C.Y.; Shen, Y.Y.; Tsai, H.D. Use of autologous buccal mucosa for vaginoplasty: A study of eight cases. Hum. Reprod. 2003, 18, 604–607.
- Li, F.Y.; Xu, Y.S.; Zhou, C.D.; Zhou, Y.; Li, S.K.; Li, Q. Long-term outcomes of vaginoplasty with autologous buccal micromucosa. Obstet. Gynecol. 2014, 123, 951–956.
- De Filippo, R.E.; Yoo, J.J.; Atala, A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003, 9, 301–306.
- De Filippo, R.E.; Bishop, C.E.; Filho, L.F.; Yoo, J.J.; Atala, A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation 2008, 86, 208–214.
- Panici, P.B.; Maffucci, D.; Ceccarelli, S.; Vescarelli, E.; Perniola, G.; Muzii, L.; Marchese, C. Autologous in vitro cultured vaginal tissue for vaginoplasty in women with Mayer-Rokitansky-Kuster-Hauser syndrome: Anatomic and functional results. J. Minim. Invasive Gynecol. 2015, 22, 205–211.
- Nodale, C.; Vescarelli, E.; D’Amici, S.; Maffucci, D.; Ceccarelli, S.; Monti, M.; Benedetti Panici, P.; Romano, F.; Angeloni, A.; Marchese, C. Characterization of human vaginal mucosa cells for autologous in vitro cultured vaginal tissue transplantation in patients with MRKH syndrome. Biomed. Res. Int. 2014, 2014, 201518.
- Zhang, J.K.; Du, R.X.; Zhang, L.; Li, Y.N.; Zhang, M.L.; Zhao, S.; Huang, X.H.; Xu, Y.F. A new material for tissue engineered vagina reconstruction: Acellular porcine vagina matrix. J. Biomed. Mater. Res. A 2017, 105, 1949–1959.
- Raya-Rivera, A.M.; Esquiliano, D.; Fierro-Pastrana, R.; Lopez-Bayghen, E.; Valencia, P.; Ordorica-Flores, R.; Soker, S.; Yoo, J.J.; Atala, A. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet 2014, 384, 329–336.
- Navarro, V.; Acien, M.I.; Acien, P. Classical mcindoe technique versus the mcindoe technique with a neovaginal paciena prosthesis((R)) and no skin graft. J. Clin. Med. 2020, 9, 3648.
- Acien, P.; Nohales-Alfonso, F.J.; Sanchez-Ferrer, M.L.; Sanchez-Lozano, M.; Navarro-Lillo, V.; Acien, M. Clinical pilot study to evaluate the neovaginal PACIENA prosthesis(R) for vaginoplasty without skin grafts in women with vaginal agenesis. BMC Womens Health 2019, 19, 144.
- Vatsa, R.; Bharti, J.; Roy, K.K.; Kumar, S.; Sharma, J.B.; Singh, N.; Singhal, S.; Meena, J. Evaluation of amnion in creation of neovagina in women with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil. Steril. 2017, 108, 341–345.
- Zhang, X.; Qiu, J.; Ding, J.; Hua, K. Comparison of neovaginoplasty using acellular porcine small intestinal submucosa graft or Interceed in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. Arch. Gynecol. Obstet. 2019, 300, 1633–1636.
- Ma, Y.; Zhang, Y.; Chen, J.; Li, L.; Liu, X.; Zhang, L.; Ma, C.; Wang, Y.; Tian, W.; Song, X.; et al. Mesenchymal stem cell-based bioengineered constructs enhance vaginal repair in ovariectomized rhesus monkeys. Biomaterials 2021, 275, 120863.
- Tian, Y.; Zhao, S.; Zheng, J.; Li, Z.; Hou, C.; Qi, X.; Kong, D.; Zhang, J.; Huang, X. A stereological study of 3D printed tissues engineered from rat vaginas. Ann. Transl. Med. 2020, 8, 1490.
- Wang, W.; Chen, F.; Cheng, J.; Peng, S.; Ye, H. Effects of different vaginal mould use approaches after vaginoplasty with artificial dermis in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. J. Int. Med. Res. 2021, 49, 300060521990519.
- Orabi, H.; Saba, I.; Rousseau, A.; Bolduc, S. Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl. Res. 2017, 180, 22–36.
- Jakubowska, W.; Chabaud, S.; Saba, I.; Galbraith, T.; Berthod, F.; Bolduc, S. Prevascularized tissue-engineered human vaginal mucosa: In vitro optimization and in vivo validation. Tissue Eng. Part A 2020, 26, 811–822.
- Switzer, B.R.; Summer, G.K. Collagen synthesis in human skin fibroblasts: Effects of ascorbate, -ketoglutarate and ferrous ion on proline hydroxylation. J. Nutr. 1972, 102, 721–728.
- Hata, R.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell Physiol. 1989, 138, 8–16.
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56.
- Michel, M.; L’Heureux, N.; Pouliot, R.; Xu, W.; Auger, F.A.; Germain, L. Characterization of a new tissue-engineered human skin equivalent with hair. Vitro Cell Dev. Biol. Anim. 1999, 35, 318–326.
- Vermette, M.; Trottier, V.; Menard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860.
- Proulx, S.; d’Arc Uwamaliya, J.; Carrier, P.; Deschambeault, A.; Audet, C.; Giasson, C.J.; Guerin, S.L.; Auger, F.A.; Germain, L. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol. Vis. 2010, 16, 2192–2201.
- Hayward, C.J.; Fradette, J.; Galbraith, T.; Rémy, C.J.; Guignard, R.; Gauvin, R.; Germain, L.; Auger, F.A. Harvesting the potential of the human umbilical cord: Isolation and characterisation of four cell types for tissue engineering applications. Cells Tissues Organs 2013, 197, 37–54.
- Magnan, M.; Berthod, F.; Champigny, M.F.; Soucy, F.; Bolduc, S. In vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J. Pediatr. Urol. 2006, 2, 261–270.
- Carrier, P.; Deschambeault, A.; Audet, C.; Talbot, M.; Gauvin, R.; Giasson, C.J.; Auger, F.A.; Guerin, S.L.; Germain, L. Impact of cell source on human cornea reconstructed by tissue engineering. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2645–2652.
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2016, 34, 121–130.
- Saba, I.; Barat, C.; Chabaud, S.; Reyjon, N.; Leclerc, M.; Jakubowska, W.; Orabi, H.; Lachhab, A.; Pelletier, M.; Tremblay, M.J.; et al. Immunocompetent human 3D organ-specific hormone-responding vaginal mucosa model of HIV-1 infection. Tissue Eng. Part C Methods 2021, 27, 152–166.
- Chabaud, S.; Rousseau, A.; Marcoux, T.L.; Bolduc, S. Inexpensive production of near-native engineered stromas. J. Tissue Eng. Regen. Med. 2017, 11, 1377–1389.
- Larouche, D.; Paquet, C.; Fradette, J.; Carrier, P.; Auger, F.A.; Germain, L. Regeneration of skin and cornea by tissue engineering. Methods Mol. Biol. 2009, 482, 233–256.
- Bouhout, S.; Perron, E.; Gauvin, R.; Bernard, G.; Ouellet, G.; Cattan, V.; Bolduc, S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng. Part A 2010, 16, 1539–1548.
- Chabaud, S.; Marcoux, T.L.; Deschenes-Rompre, M.P.; Rousseau, A.; Morissette, A.; Bouhout, S.; Bernard, G.; Bolduc, S. Lysophosphatidic acid enhances collagen deposition and matrix thickening in engineered tissue. J. Tissue Eng. Regen. Med. 2015, 9, E65–E75.
- Galbraith, T.; Roy, V.; Bourget, J.M.; Tsutsumi, T.; Picard-Deland, M.; Morin, J.F.; Gauvin, R.; Ismail, A.A.; Auger, F.A.; Gros-Louis, F. Cell seeding on UV-C-treated 3D polymeric templates allows for cost-effective production of small-caliber tissue-engineered blood vessels. Biotechnol. J. 2019, 14, e1800306.
- Magnan, M.; Levesque, P.; Gauvin, R.; Dube, J.; Barrieras, D.; El-Hakim, A.; Bolduc, S. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng. Part A 2009, 15, 197–202.
- Imbeault, A.; Bernard, G.; Rousseau, A.; Morissette, A.; Chabaud, S.; Bouhout, S.; Bolduc, S. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can. Urol. Assoc. J. 2013, 7, E4–E9.
- Cattan, V.; Bernard, G.; Rousseau, A.; Bouhout, S.; Chabaud, S.; Auger, F.A.; Bolduc, S. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur. Urol. 2011, 60, 1291–1298.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
735
Revisions:
2 times
(View History)
Update Date:
26 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




