You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Stéphane Bolduc and Version 2 by Lily Guo.
Tissue engineering is an emerging field of research that initially aimed to produce 3D tissues to bypass the lack of adequate tissues for the repair or replacement of deficient organs.
- tissue engineering
- urethra
- vagina
- urology
- gynaecology
- epithelium
1. Introduction
Despite the significant advances in medicine of the last decades, many illnesses are still challenging. Indeed, the treatments themselves can be insufficient, or their side effects may negatively impact the patient’s quality of life. From congenital anomalies to various injuries or illnesses that may develop, the list goes on and can range from a simple annoyance to a deadly threat. Affectations of the genitourinary tissues are no exception in this respect and can significantly contribute to undermining the social and emotional life of individuals [1][2][3][4][5][1,2,3,4,5]. In this context, a particular focus will be placed on the male urethra and the vagina. This review examines the anatomy, pathologies and treatments. Current treatments will be studied, as well as encountered problems and possible solutions offered by tissue engineering. The field of tissue engineering has emerged in the past decades, aiming to treat or replace damaged tissues. Various approaches will be described, and some of their applications either repair the urethra and the vagina or create study models. A new and exceptionally innovative approach will then be put forward: The self-assembly technique [6].
2. Tissue Engineering
In 1993, Langer and Vacanti published a paper in Science entitled “Tissue Engineering” (TE) [7][67]. This was the first assay to conceptualise the notion of TE and its goals. From this date, TE has been considerably developed to offer new and innovative solutions to old problems. In the context of ageing and the sedentary behaviour of human populations, the appearance of chronic diseases that require organ repair or replacement is increasing even though the number of organs available for transplants is reducing precisely because of this ageing and sedentary behaviour, but also the strengthening of selection criteria by regulatory agencies. Tissue engineering consists of the in vitro reconstruction of tissues or organs for the replacement/repair and 3D models for fundamental research. Synthetic or natural biomaterials, including decellularised organs as scaffolds, seeded or not with cells, have been studied. Various techniques have been described to create the scaffold, such as casting [8][68], electrospinning [9][10][11][69,70,71] or bioprinting [12][72]. Other methods like the self-assembly technique, which does not require any exogenous biomaterial, have also been developed.
2.1. Synthetic Materials
Synthetic biomaterials present many advantages, such as their low cost, ability to be highly tunable and form the desired geometry [13][14][15][73,74,75]. They also present some drawbacks, such as their difference in composition and organisation compared to a natural extracellular matrix (ECM), which can impact adhesion, proliferation and differentiation of cells [16][76]. Indeed, the low diversity of the components used to produce these biomaterials appears simplistic compare to the numerous components of a native ECM. Nevertheless, significant progress has been made with the functionalisation of synthetic biomaterials in the last decades. For many applications, the use of hydrogels is interesting, and recent studies have shown a potential for them functionalised with different peptides to recreate, at least in part, the microenvironment of the target tissues [17][77]. This kind of functionalised hydrogels could support cell growth/differentiation, as well as vehicles for the delivery of stem cells, drugs or bioactive proteins.
2.2. Natural Materials
Contrarily to synthetic materials, natural biomaterials used as scaffolds, especially those using elements coming from ECM [18][78], provide better adapted environments for migration, proliferation and differentiation of cells when used under appropriate conditions. Nevertheless, these biomaterials generally have weaker mechanical properties compared to native ECM. Indeed, the mechanical properties of ECM are not only due to the molecules it is composed of, but also, more importantly, because the organisation of these molecules in the matrix.
Among other promising biomaterials, decellularised tissues are becoming more and more attractive with new protocols of decellularisation [19][79]. Indeed, it seems convenient to use the complexity of native materials to repair themselves. Decellularising tissues to remove antigenic molecules responsible for immunological response and potential pathogens before being recellularised with patient’s cells allows tissue production, which is very close to the autograft or even better if the disease’s causal effect is also corrected. Decellularisation of tissue can be obtained by successive hypo/hypertonic shocks, combined or not with the addition of detergents, to destroy the cell membrane [20][80]. Enzymes can be used to remove proteins and nucleic acids. The tissue can originate from a human cadaver or be obtained from animals.
Nevertheless, several problems slow down the use of such prostheses: Ethical problems and various regulatory issues, such as the risk of immune reaction in ineffective/incomplete decellularisation. However, the main problem remains the ability to maintain the ECM architecture that provides good mechanical and biological characteristics to the graft. Indeed, heavy decellularisation can induce a loss of the native matrix architecture and its non-immunogen components, whereas weak decellularisation could be insufficient to obtain a safe biomaterial [21][22][23][81,82,83]. In the past, ECM was largely disorganised or modified by the decellularisation process, losing the mechanical properties, resistance, elasticity and cell differentiation potential. An increasing number of protocols are emerging to reduce these problems, but more studies are needed to obtain a clear view for this option [24][84]. Recently a novel approach to functionalise decellularised tissues has been proposed to improve endothelial cell adhesion and accelerates endothelialisation, which is an important point, due to the need to rapidly provide oxygen and nutrients to grafts to ensure positive outcomes. This goal is achieved through selective immobilisation of REDV tetrapeptides [25][85].
2.3. Tissue Engineering for Urethral Reconstruction
Several groups have attempted urethral substitution using TE cell-free matrices, such as bladder acellular matrix graft (BAMG) and small intestinal submucosa (SIS) or cellularised matrices [26][27][28][29][30][31][32][33][86,87,88,89,90,91,92,93]. These matrices are prepared from native tissues by decellularising and sterilising them. As shown in rabbits by Dorin et al., a significant problem of acellular matrices is that urothelial regeneration is limited to 0.5 cm, which compromises success in more complex cases, such as long strictures [34][94]. Synthetic polymers have also shown advantages (poly-l-lactic acid, (PLLA) and poly(lactic-co-glycolic) acid, (PLGA)) for forming low-cost, biocompatible, three-dimensional (3D) organs with controlled mechanical properties. However, synthetic scaffolds without functionalisation by peptides do not allow the proper differentiation of epithelial cells into well-organised tissue. Indeed, contrarily to natural matrices, they cannot recreate the target organ microenvironment, especially adequate ECM-cell interaction (e.g., lack integrin-binding peptide sequence, failure in synchronisation between degradation rate and matrix neo-deposition) [13][35][36][73,95,96]. No long-term experiment has been performed with a significant number of patients. Currently, protocols developed are not used in clinics despite the media coverage of some, signalling the immaturity of the works, which must continue to be improved [37][97]. TE substitutes that contain autologous cells in addition to an extracellular matrix, close to the native one, are more promising. The main advantage of this method is that a large graft of autologous cells can be produced with a limited sample, such as a piece of oral or bladder mucosa. Indeed, the extracted cells can be grown in vivo, seeded on the biomaterial and implanted with a very low risk of rejection. Studies have also reported that stem cells can be obtained from urine, making this approach potentially useful [38][39][98,99]. A downfall of this method is that after long periods of culture to obtain well-differentiated tissues, the exogenous matrices become challenging to manipulate and lose their mechanical and physical properties. Despite significant progress in urethral TE, very few teams have performed clinical trials and published their results to date [40][100] (Table 1). However, the four clinical trials conducted to date show promising results in a limited number of patients with long-segment and/or complex stenosis [37][41][42][43][44][97,101,102,103,104]. While these models are certainly far from a “plug and play” alternative with consistently reproducible results, they could offer an alternative for complex cases requiring long segment urethral replacement [45][105].
Table 1.
The scaffold used for urethral reconstruction.
| Type of Scaffold | Biomaterials | Ref Example | Advantages | Drawbacks | |
|---|---|---|---|---|---|
| Synthetic | PLCL | [46] | [106] | - biocompatible - mechanical properties |
- Degradation products |
| PLCL/Collagen | [47] | [107] | - low cost | - Poor differentiation of epithelial cells (except for cellularised collagen matrices; improved by functionalisation) | |
| PLA | [48] | [108] | - highly reproducible | -degradation rate (too low or too high) | |
| PU/mesh in PGA | [49] | [109] | - quickly available | ||
| Self-Assembly | |||||
| None | |||||
| [ | |||||
| 13 | |||||
| ] | |||||
| [ | |||||
| 71 | ] | [72] | [73,131,132] | - Excellent microenvironment with organ-specific cells - Mechanical properties |
- time and cost to produce tissues |
2.4. Tissue Engineering for Vaginal Reconstruction
Vaginal abnormalities represent a significant health problem for women because nearly 1% of women will suffer from the pathologies mentioned previously, resulting in significant psychological impacts. Interestingly, tissue engineering is an area that aims to replace or regenerate dysfunctional tissues and organs with autologous cells, biomaterials or a combination of both. The success of vaginal reconstruction in these patients largely depends on the use of a sufficiently large tissue substrate that adequately fulfils the physiological functions of the vagina. Prior techniques have often relied on autologous tissues, such as the intestine or skin, often associated with complications, due to the physiological differences inherent to these substrates. To improve results, a variety of biodegradable substitutes, including collagen matrices and a decellularised bladder submucosa, have been used for vaginal replacement [42][73][102,134]. Reconstructions using these substitutes have generally failed, due to functional, structural, mechanical or biocompatibility issues. Using the patient’s vaginal tissue for reconstruction would be the most elegant and effective solution, but this has often not been possible, due to the relative scarcity of healthy vaginal tissue for autologous transplantation. There is a substantial clinical need to develop technologies to facilitate the regeneration of injured or diseased tissues and organs. The relentless prevalence of trauma, congenital abnormalities and diseases, such as cancer, is driving demand, becoming increasingly urgent as the world’s population grows and ages. A wide variety of tissues and organs would benefit from engineering-based repairing or regeneration. Several graft materials have been used to line the surgically created neovaginal cavity, including myocutaneous flaps or intestinal segments, full-thickness or split-thickness skin grafts, amniotic membrane, peritoneum, buccal mucosa and vaginal epithelial tissue [74][75][76][77][78][79][80][81][82][135,136,137,138,139,140,141,142,143]. These techniques are associated with contraction and/or stenosis of the graft, which may require long-term dilation. Oral mucosa vaginoplasties are associated with donor site morbidities, due to the large volume of tissue taken to create the neovagina. In addition, the amount of tissue that can be harvested from a donor site is limited, which can be problematic, especially for significant defects. To overcome these difficulties, alternative methods of vaginal reconstruction have been explored. Few groups have attempted TE vaginal reconstruction using acellular and cellular matrices of natural or synthetic origin [83][84][85][86][87][88][144,145,146,147,148,149] (Table 2). The tissues were transplanted into mice, rabbits and humans. However, further preclinical and clinical studies are needed, due to the limited number of subjects included in these studies. It remains challenging to determine whether the optimal technique was used.
Table 2. The scaffold used for vaginal reconstruction.
| Type of Scaffolds | Biomaterials | Patients # | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic | PGA | 4 | [88] | [149] | |||||||||
| PLA (©PACIENA) | 9 | [89] | [150] | ||||||||||
| 7 | [90] | [151] | |||||||||||
| Natural | Collagen IV and hyaluronic acid | 1 | [85] | -mechanical properties during or after degradation | |||||||||
| [ | 146 | ] | PLGA | [37 | |||||||||
| ] | [97] | - functionalisation | 23 | - poor angiogenesis | |||||||||
| [ | 85 | ] | [146] | PLLA | [50] | [110] | |||||||
| Acellular matrix | Amnion | 50 | [91] | [152] | Natural | Cellulose | [51] | [111] | |||||
| SIS | 65 (vs Interceed) | [92] | [153] | Silk Fibroin | [26][52][53][54] | [86,112,113,114] | |||||||
| Monkey | [93] | [154] | Collagen | [18][28][55][56][57][58][59] | [78,88,115,116,117,118,119] | ||||||||
| Acellular vaginal matrix | Rat | [94] | [155] | Acellular matrix | SIS | [21] | 81 | [60][61][62][ | ,120 | 63 | |||
| , | 121 | ] | ,122 | [64 | ,123 | ][65] | [,124,125] | - Adequate microenvironment for cell proliferation and differentiation | Rat | [87 | - Immune risk (including DNA, prions) | ||
| ] | [ | 148 | ] | Placental membrane | [66] | [126] | - Significant angiogenesis | - Unfavourable clinical experience | |||||
| Artificial dermis | 35 | [95] | [156] | BAMG | [67][68][69] | [127,128,129] | - Quality of the matrix | ||||||
| Self-Assembly | Mouse | [96][97] | [157,158] | Urethra | [70] | [130] |
3. The Self-Assembly Approach
New approaches are required to combine adequate cell signalling and differentiation, cell maintenance, especially for stem cells, and sufficient mechanical resistance for implantation. All this while minimising side effects. A new type of strategy has been explored by the group of Dr. François A. Auger at LOEX: The “self-assembly” method [6]. Great discoveries and therapeutic achievements have been made possible thanks to this unique technique which allows the production of reconstructed tissues free of exogenous materials. Indeed, the use of exogenous biomaterials can lead to immunological, foreign body reactions and the transmission of infections. This technique relies on cells cultured in ascorbic acid to secrete and deposit their own ECM to form cohesive sheets of cells and collagen [98][99][159,160]. While most biomaterials lose their mechanical and physical strength properties in culture, these self-assembled tissue properties are roughly similar or even exceeding those of native tissues in some models, due to the stabilisation of metalloproteinases [100][161]. The self-assembly technique has made it possible to construct models from various stromal cells of the skin [101][162], fat [102][163], cornea [103][164], Warton’s jelly [104][165], bladder [105][166] and vagina [96][157] exhibit not only excellent mechanical strength, but also an allowance for adequate epithelial differentiation.
The first step of a tissue’s reconstruction using the self-assembly technique is to seed mesenchymal cells and cultivate them in the presence of ascorbate, also called vitamin C. It is preferable to use organ-specific mesenchymal cells (e.g., dermal fibroblasts to reconstruct skin substitutes, keratocytes to reconstruct cornea substitutes [106][167], bladder mesenchymal cells to reconstruct bladder mucosa substitutes [107][133] and vagina fibroblasts to reconstruct vaginal mucosa substitutes [108][168]). The use of unpaired mesenchymal cells can result in inadequate differentiation. Indeed, cutaneous differentiation of corneal or urothelial epithelial cells occurs when these cells are cultivated on dermal fibroblasts-derived stromas. The ascorbate concentration was set at 50 µg/mL even if it is not the optimal concentration for collagen deposition in dermal fibroblast cell culture. Indeed, higher concentrations of this oxidative agent induce cell death, and thus, reduce the total amount of deposited collagen and the mechanical strength of the final product. After 3 to 6 weeks, depending on the ability of the cultivated cells to deposit ECM, the mesenchymal cells have produced a thick material similar to a native stroma of the target organ (Figure 1). It is possible to refine the protocol by introducing, for example, at the initial seeding step, endothelial cells from an umbilical vein or, even better, from the organ-specific microvascular network to form a capillary-like network [109][169] or immune cells, such as monocyte-derived macrophages to produce immunocompetent models [108][168]. A reseeding step can also be done after two weeks to increase the tissue thickness and improve the cell distribution inside the stroma, which can especially be helpful for the microvascular network and increase elastic properties of the models [109][169] (Figure 2A). Following the step of stroma production, epithelial cells can be seeded on the top of the construct and cultivated for one week in submerged conditions, allowing the complete coverage of its upper surface before being placed at the air/liquid interface for three weeks to obtain differentiation of the epithelium. This technique has shown a high level of differentiation in various models, showing epitheliums very similar to native tissues. To place bladder tissues in physiological conditions, a bioreactor has been designed (Figure 2B). Detailed protocols can be found in several research articles and reviews [110][170].
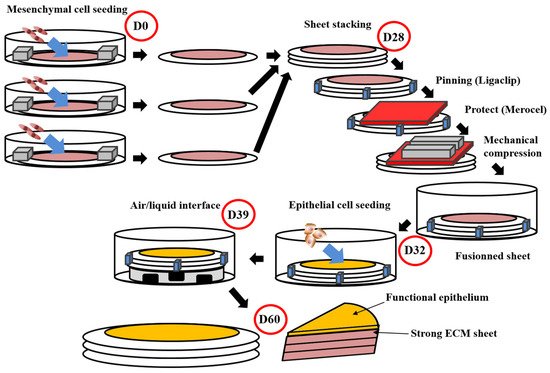
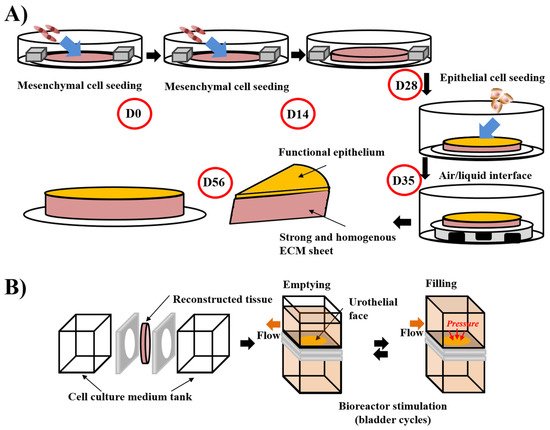

Figure 1. Schema of the self-assembly method using the standard protocol. This protocol obtains flat tissues with functional epithelium and a strong ECM sustaining manipulations by surgeons or researchers [105][111][112][166,171,172]. The total time of production around 60 days. For the whole process, media are supplemented with ascorbate (50 µg/mL). On day 0 (D0), mesenchymal cells are seeded into petri dishes containing a paper ring as an anchorage device weighted by small metal lingots. Cells are then cultured for 28 days. On day 28 (D28), stroma sheet (cells + ECM) are stacked (air bubble must be avoided between sheets), then they are pinned together using a surgical clip (Ligaclip), covered by a surgical sponge (Merocel) to protect from the direct contact with metal lingots use to favour fusion through mechanical compression. Culture is pursued for 4 days until day 32 (D32) to ensure adequate fusion. Then epithelial cells are seeded on the top of the construct and cultured for a week to allow horizontal coverage of the scaffold. On day 39 (D39), the constructs are raised at the air/liquid interface using a specific device allowing media circulation under the reconstructed tissue. This phase allows the maturation of epithelium until day 60 (D60).

Figure 2. Schema of the “reseeding” variation of the self-assembly protocol and the stimulation of bladder mucosa substitute in a bioreactor. (A) The “reseeding” protocol reduces the cost and the complexity of the self-assembly technique compared to the standard protocol, avoiding the need for multiple sheets and their stacking. A better distribution of the cells throughout the tissue has also been demonstrated. (B) Stimulation of bladder mucosa substitute using bioreactor. The substitute is inserted between two chambers where the cell culture medium can circulate. To mimic the bladder cycles of emptying and filling, the flow is modified and increased the pressure on the urothelial face of the substitute in the filled status, whereas the substitute is more relaxed in the empty condition.
For tubular substitutes, such as blood vessels or urethras, the epithelial/endothelial seeding does not happen immediately after the step of stroma production. The tissue-like structure is detached from the petri dish and tightly rolled around a mandrel of the appropriate diameter [100][161]. After the rolling step, the fusion of the rolls was helped by maintaining a mechanical load. The cylindrical mandrel can then be removed from the tubular structure, creating a lumen. The lumen is filled with liquid to avoid collapse, and epithelial/endothelial cells can be seeded inside the tube for urethral or blood vessel reconstruction, respectively. During the epithelial/endothelial cell seeding step, rotation of the tube ensures a uniform distribution of these cells (Figure 3A). Once again, urologic tissues can be matured under physiological conditions using bioreactors (Figure 3B). Various refinement has been introduced for these constructs. Notably, mesenchymal cells can be seeded directly on the mandrel to form the tubular structure, avoiding delamination of the rolls in the case of their incomplete fusion [113][173].
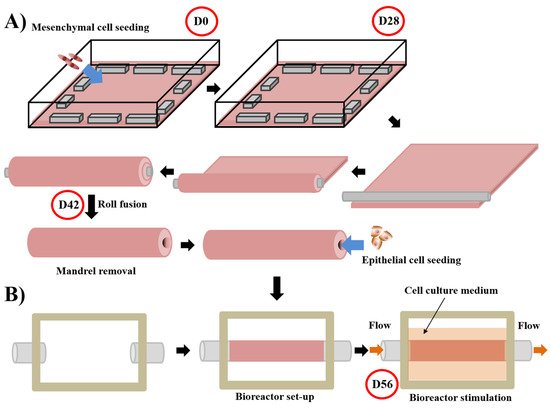

Figure 3. Schema of the technique used to produce tubular substitute using the self-assembly approach and the subsequent stimulation in a bioreactor of the substitutes. For the whole process, media are supplemented with ascorbate (50 µg/mL). (A) Mesenchymal cells are seeded at day 0 (D0) on a gelatin-coated square plate and cultured for 28 days. Contraction of the tissue is avoided by putting flat metal lingots on its border. After the mesenchymal cells formed an ECM sheet (day 28 (D28)), the latter was rolled tightly around a cylindrical mandrel. Fusion of the rolls is allowed by maintaining a little load on the tissue for 14 days. On day 42 (D42), the mandrel can be removed and epithelial cells seeded inside the tubular structure [114][115][174,175]. (B) The day after, a bioreactor separates the external and internal parts of the tissue, a flow circulating in the internal part mimicking the physiological flow. The cell culture medium is present in the external part to provide nutrients. Maturation is done for 14 days until day 56 (D56) [116][176].
4. Perspectives
The field of tissue engineering can bring potential alternatives to the constantly increasing need for tissue repair/replacement of urological organs. To do so, a real challenge is to combine good mechanical properties while maintaining stem cell potential. It must also allow adequate epithelium differentiation, and its degradation product should not imply adverse effects. It must be vascularised easily and not represent an immune risk for patients, while being functional as soon as the graft is in place. We can, therefore, better understand the significant obstacle that must be overcome.
In this article, we detailed the self-assembly technique, without using synthetic or decellularised biomaterials/scaffolds. Indeed, genitourinary tissues have been developed using animal cells and have been successfully implanted in animals. To facilitate clinical translation and avoid interspecies differences, the next step is to graft human organ-specific tissues in immunosuppressed animals before trying the prototype on human subjects. These tissues constitute a promising avenue for the surgical correction of various defects, whether from congenital or acquired origin.
Several new challenges emerge, such as urothelial, mesenchymal and endothelial cells differentiated from iPSC derived from blood cells. This would avoid the need for a biopsy with its potential comorbidities, and avoid the problem of the non-organ-specific communications between cells leading to aberration or the presence of inadequate cells to harvest (e.g., cancer). However, there remains a lot to be done to obtain an adequate differentiation from iPSC without aberrant expression of oncogenes. Another challenge in the coming years should be to exclude the use of serum in cell culture. Serum could induce a loss of reproducibility in some experiments and be a source of contamination and raise ethical concerns. Furthermore, the world’s increase in demand will keep dragging the price up while the global production of serum is stagnant.
