
Video Upload Options
In the last decades, a kind of small non-coding RNA molecules, called as microRNAs, has been applied as negative regulators in various types of cancer treatment through down-regulation of their targets. More recent studies exert that microRNAs play a critical role in the EMT process of cancer, promoting or inhibiting EMT progression. Interestingly, accumulating evidence suggests that pure compounds from natural plants could modulate deregulated microRNAs to inhibit EMT, resulting in the inhibition of cancer development. This small essay is on the purpose of demonstrating the significance and function of microRNAs in the EMT process as oncogenes and tumor suppressor genes according to studies mainly conducted in the last four years, providing evidence of efficient target therapy.
1. Introduction
2. OncomiRs in EMT
2.1. Breast Cancer
2.2. Colorectal Cancer
2.3. Cervical Cancer
2.4. Gastric Cancer
2.5. Nasopharyngeal Carcinoma
2.6. Lung Cancer
2.7. Osteosarcoma
2.8. Other Types of Cancer
3. TsmiRs in EMT
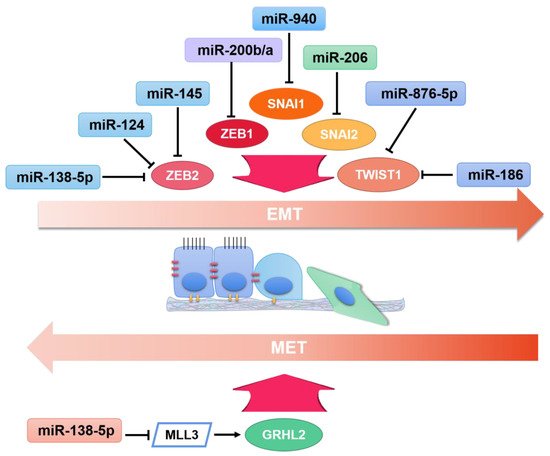
3.1. Breast Cancer
3.2. Colorectal Cancer
3.3. Gastric Cancer
3.4. Glioma
3.5. Lung Cancer
3.6. Pancreatic Cancer
3.7. Papillary Thyroid Cancer
3.8. Other Types of Cancer
References
- Demirkan, B. The Roles of Epithelial-to-Mesenchymal Transition (EMT) and Mesenchymal-to-Epithelial Transition (MET) in Breast Cancer Bone Metastasis: Potential Targets for Prevention and Treatment. J. Clin. Med. 2013, 2, 264–282.
- Gupta, S.; Maitra, A. EMT: Matter of Life or Death? Cell 2016, 164, 840–842.
- Biswas, K.H. Molecular Mobility-Mediated Regulation of E-Cadherin Adhesion. Trends Biochem. Sci. 2020, 45, 163–173.
- Nakagawa, M.; Bando, Y.; Nagao, T.; Morimoto, M.; Takai, C.; Ohnishi, T.; Honda, J.; Moriya, T.; Izumi, K.; Takahashi, M.; et al. Expression of p53, Ki-67, E-cadherin, N-cadherin and TOP2A in triple-negative breast cancer. Anticancer Res. 2011, 31, 2389–2393.
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972.
- Alves, C.C.; Carneiro, F.; Hoefler, H.; Becker, K.F. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front. Biosci. 2009, 14, 3041–3050.
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch. Pharmacal Res. 2019, 42, 14–24.
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94.
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499.
- Chung, V.Y.; Tan, T.Z.; Ye, J.R.; Huang, R.L.; Lai, H.C.; Kappei, D.; Wollmann, H.; Guccione, E.; Huang, R.Y.J. The role of GRHL2 and epigenetic remodeling in epithelial-mesenchymal plasticity in ovarian cancer cells. Commun. Biol. 2019, 2, 15.
- Mooney, S.M.; Talebian, V.; Jolly, M.K.; Jia, D.; Gromala, M.; Levine, H.; McConkey, B.J. The GRHL2/ZEB Feedback Loop-A Key Axis in the Regulation of EMT in Breast Cancer. J. Cell. Biochem. 2017, 118, 2559–2570.
- Li, S.; Yang, J. Ovol Proteins: Guardians against EMT during Epithelial Differentiation. Dev. Cell 2014, 29, 1–2.
- Khoshnaw, S.M.; Green, A.; Powe, D.G.; Ellis, I. MicroRNA involvement in the pathogenesis and management of breast cancer. J. Clin. Pathol. 2009, 62, 422–428.
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060.
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Radmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419.
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. miRNA-Based Therapeutics in Breast Cancer: A Systematic Review. Front. Oncol. 2021, 11, 14.
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14.
- Berindan-Neagoe, I.; Calin, G.A. Molecular Pathways: MicroRNAs, Cancer Cells, and Microenvironment. Clin. Cancer Res. 2014, 20, 6247–6253.
- D’Amato, N.C.; Howe, E.N.; Richer, J.K. MicroRNA regulation of epithelial plasticity in cancer. Cancer Lett. 2013, 341, 46–55.
- Tang, J.; Li, Y.; Wang, J.; Wen, Z.; Lai, M.; Zhang, H. Molecular mechanisms of microRNAs in regulating epithelial–mesenchymal transitions in human cancers. Cancer Lett. 2016, 371, 301–313.
- Peng, F.; Xie, X.; Peng, C. Chinese Herbal Medicine-Based Cancer Therapy: Novel Anticancer Agents Targeting MicroRNAs to Regulate Tumor Growth and Metastasis. Am. J. Chin. Med. 2019, 47, 1711–1735.
- Ahmed, F.; Ijaz, B.; Ahmad, Z.; Farooq, N.; Sarwar, M.B.; Husnain, T. Modification of miRNA Expression through plant extracts and compounds against breast cancer: Mechanism and translational significance. Phytomedicine 2020, 68, 153168.
- Sreekumar, R.; Sayan, B.S.; Mirnezami, A.H.; Sayan, A.E. MicroRNA Control of Invasion and Metastasis Pathways. Front. Genet. 2011, 2, 58.
- Jiang, G.B.; Shi, W.W.; Fang, H.Y.; Zhang, X.H. miR-27a promotes human breast cancer cell migration by inducing EMT in a FBXW7-dependent manner. Mol. Med. Rep. 2018, 18, 5417–5426.
- Wang, Z.; Wang, X.Y. miR-122-5p promotes aggression and epithelial-mesenchymal transition in triple-negative breast cancer by suppressing charged multivesicular body protein 3 through mitogen-activated protein kinase signaling. J. Cell. Physiol. 2020, 235, 2825–2835.
- Liu, X.Y.; Li, Y.J.; Li, Z.; Hou, T. miR-155 promotes proliferation and epithelial-mesenchymal transition of MCF-7 cells. Exp. Ther. Med. 2021, 21, 7.
- Lei, B.; Wang, D.D.; Zhang, M.; Deng, Y.W.; Jiang, H.J.; Li, Y.W. miR-615-3p promotes the epithelial-mesenchymal transition and metastasis of breast cancer by targeting PICK1/TGFBRI axis. J. Exp. Clin. Cancer Res. 2020, 39, 14.
- Hu, Y.; Su, Y.; He, Y.; Liu, W.; Xiao, B. Arginine methyltransferase PRMT3 promote tumorigenesis through regulating c-MYC stabilization in colorectal cancer. Gene 2021, 791, 145718.
- Ma, Z.H.; Shi, P.D.; Wan, B.S. MiR-410-3p activates the NF-kappa B pathway by targeting ZCCHC10 to promote migration, invasion and EMT of colorectal cancer. Cytokine 2021, 140, 10.
- Liu, D.S.; Zhang, H.; Cui, M.M.; Chen, C.S.; Feng, Y. Hsa-miR-425-5p promotes tumor growth and metastasis by activating the CTNND1-mediated beta-catenin pathway and EMT in colorectal cancer. Cell Cycle 2020, 19, 1917–1927.
- Wang, H.; Yan, B.; Zhang, P.; Liu, S.; Li, Q.; Yang, J.; Yang, F.; Chen, E. MiR-496 promotes migration and epithelial-mesenchymal transition by targeting RASSF6 in colorectal cancer. J. Cell. Physiol. 2020, 235, 1469–1479.
- Zhu, J.; Han, S. miR-150-5p promotes the proliferation and epithelial-mesenchymal transition of cervical carcinoma cells via targeting SRCIN1. Pathol. Res. Pr. 2019, 215, 738–747.
- Qu, D.; Yang, Y.; Huang, X. miR-199a-5p promotes proliferation and metastasis and epithelial-mesenchymal transition through targeting PIAS3 in cervical carcinoma. J. Cell. Biochem. 2019, 120, 13562–13572.
- Xiao, T.; Jie, Z. MiR-21 Promotes the Invasion and Metastasis of Gastric Cancer Cells by Activating Epithelial-Mesenchymal Transition. Eur. Surg. Res. 2019, 60, 208–218.
- Zhang, Y.; Meng, W.; Yue, P.; Li, X. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J. Exp. Clin. Cancer Res. 2020, 39, 1–20.
- Ma, R.; Shimura, T.; Yin, C.; Okugawa, Y.; Kitajima, T.; Koike, Y.; Okita, Y.; Ohi, M.; Uchida, K.; Goel, A.; et al. Antitumor effects of Andrographis via ferroptosis-associated genes in gastric cancer. Oncol. Lett. 2021, 22, 1–8.
- Wu, Z.-H.; Lin, C.; Liu, C.-C.; Jiang, W.-W.; Huang, M.-Z.; Liu, X.; Guo, W.-J. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 501, 1068–1073.
- Chen, H.; Luo, M.; Wang, X.; Liang, T.; Huang, C.; Huang, C.; Wei, L. Inhibition of PAD4 enhances radiosensitivity and inhibits aggressive phenotypes of nasopharyngeal carcinoma cells. Cell. Mol. Biol. Lett. 2021, 26, 1–12.
- Cheng, Z.Q.; Qiang, H.L.; Wang, B.; Ding, Z.R.; Jiang, C.Y. mir-10b modulates the epithelial-mesenchymal transition, proliferation and migration of nasopharyngeal carcinoma cells. Acta Medica Mediterr. 2020, 36, 941–945.
- Zhang, P.; Lu, X.; Shi, Z.; Li, X.; Zhang, Y.; Zhao, S.; Liu, H. miR-205-5p regulates epithelial-mesenchymal transition by targeting PTEN via PI3K/AKT signaling pathway in cisplatin-resistant nasopharyngeal carcinoma cells. Gene 2019, 710, 103–113.
- Dai, L.H.; Chen, F.H.; Zheng, Y.H.; Zhang, D.; Qian, B.; Ji, H.X.; Long, F.; Cretoiu, D. miR-21 regulates growth and EMT in lung cancer cells via PTEN/Akt/GSK3 beta signaling. Front. Biosci. 2019, 24, 1426–1439.
- Yang, F.M.; Shao, C.C.; Wei, K.; Jing, X.M.; Qin, Z.Q.; Shi, Y.N.; Shu, Y.Q.; Shen, H. miR-942 promotes tumor migration, invasion, and angiogenesis by regulating EMT via BARX2 in non-small-cell lung cancer. J. Cell. Physiol. 2019, 234, 23596–23607.
- Marina, N.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and Therapeutic Advances for Pediatric Osteosarcoma. Oncologist 2004, 9, 422–441.
- Zhao, X.; Xu, Y.; Sun, X.; Ma, Y.; Zhang, Y.; Wang, Y.; Guan, H.; Jia, Z.; Li, Y.; Wang, Y. miR-17-5p promotes proliferation and epithelial-mesenchymal transition in human osteosarcoma cells by targeting SRC kinase signaling inhibitor 1. J. Cell. Biochem. 2019, 120, 5495–5504.
- Li, F.; Chen, Q.; Yang, Y.; Li, M.; Zhang, L.; Yan, Z.; Zhang, J.; Wang, K. ESR1 as a recurrence-related gene in intrahepatic cholangiocarcinoma: A weighted gene coexpression network analysis. Cancer Cell Int. 2021, 21, 1–12.
- Tang, Y.; Yang, J.R.; Wang, Y.G.; Tang, Z.Y.; Liu, S.L.; Tang, Y.T. MiR-19b-3p facilitates the proliferation and epithelial-mesenchymal transition, and inhibits the apoptosis of intrahepatic cholangiocarcinoma by suppressing coiled-coil domain containing 6. Arch. Biochem. Biophys. 2020, 686, 11.
- Ye, Y.; Dai, Q.J.; Qi, H.B. A novel defined pyroptosis-related gene signature for predicting the prognosis of ovarian cancer. Cell Death Discov. 2021, 7, 11.
- Zhang, L.Y.; Chen, Y.; Jia, J.; Zhu, X.; He, Y.; Wu, L.M. MiR-27a promotes EMT in ovarian cancer through active Wnt/beta-catenin signalling by targeting FOXO1. Cancer Biomark. 2019, 24, 31–42.
- Coffey, K. Targeting the Hippo Pathway in Prostate Cancer: What’s New? Cancers 2021, 13, 611.
- Wang, Y.; Hu, J.D.; Qi, G.Y.; Wang, S.H.; Gao, J.J. miR-19a promotes the metastasis and EMT through CUL5 in prostate cancer cell line PC3. J. Buon 2020, 25, 2028–2035.
- Chou, S.-T.; Ho, B.-Y.; Tai, Y.-T.; Huang, C.-J.; Chao, W.-W. Bidirect effects from cisplatin combine with rosmarinic acid (RA) or hot water extracts of Glechoma hederacea (HWG) on renal cancer cells. Chin. Med. 2020, 15, 1–13.
- Lei, Q.-Q.; Huang, Y.; Li, B.; Han, L.; Lv, C. MiR-155-5p promotes metastasis and epithelial–mesenchymal transition of renal cell carcinoma by targeting apoptosis-inducing factor. Int. J. Biol. Markers 2021, 36, 20–27.
- Shen, J.L.; Lu, Y.Q.; Li, N.; Zhang, Y.; Hu, F.; Dai, H.; Cai, H.F.; Yan, J.Y. miR-34b Inhibits Breast Cancer Cell Epithelial-Mesenchymal Transition (EMT) and Invasion via Targeting Glioma-Associated Oncogene Protein 1 (Gli1). J. Biomater. Tissue Eng. 2020, 10, 1465–1470.
- Jia, H.; Sang, M.X.; Liu, F.; Ai, N.; Geng, C.Z. miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol. Res. Pract. 2019, 215, 697–704.
- Jiang, D.; Zhou, B.; Xiong, Y.; Cai, H. miR-135 regulated breast cancer proliferation and epithelial-mesenchymal transition acts by the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2019, 43, 1623–1634.
- Du, F.; Yu, L.; Wu, Y.; Wang, S.; Yao, J.; Zheng, X.; Xie, S.; Zhang, S.; Lu, X.; Liu, Y.; et al. miR-137 alleviates doxorubicin resistance in breast cancer through inhibition of epithelial-mesenchymal transition by targeting DUSP4. Cell Death Dis. 2019, 10, 1–10.
- Sun, W.J.; Zhang, Y.N.; Xue, P. miR-186 inhibits proliferation, migration, and epithelial-mesenchymal transition in breast cancer cells by targeting Twist1. J. Cell. Biochem. 2019, 120, 10001–10009.
- Min, L.; Liu, C.; Kuang, J.; Wu, X.; Zhu, L. miR-214 inhibits epithelial–mesenchymal transition of breast cancer cells via downregulation of RNF8. Acta Biochim. Biophys. Sin. 2019, 51, 791–798.
- Chi, Y.Y.; Wang, F.; Zhang, T.F.; Xu, H.; Zhang, Y.N.; Shan, Z.Z.; Wu, S.X.; Fan, Q.X.; Sun, Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J. Cell Mol. Med. 2019, 23, 6295–6307.
- Zhang, K.; Hu, Y.; Luo, N.; Li, X.; Chen, F.; Yuan, J.; Guo, L. miR-574-5p attenuates proliferation, migration and EMT in triple-negative breast cancer cells by targeting BCL11A and SOX2 to inhibit the SKIL/TAZ/CTGF axis. Int. J. Oncol. 2020, 56, 1240–1251.
- Liu, G.; Wang, P.; Zhang, H. MiR-6838-5p suppresses cell metastasis and the EMT process in triple-negative breast cancer by targeting WNT3A to inhibit the Wnt pathway. J. Gene Med. 2019, 21, e3129.
- Du, G.; Yu, X.; Chen, Y.; Cai, W. MiR-1-3p Suppresses Colorectal Cancer Cell Proliferation and Metastasis by Inhibiting YWHAZ-Mediated Epithelial–Mesenchymal Transition. Front. Oncol. 2021, 11.
- Wang, W.-X.; Yu, H.-L.; Liu, X. MiR-9-5p suppresses cell metastasis and epithelial-mesenchymal transition through targeting FOXP2 and predicts prognosis of colorectal carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6467–6477.
- Fan, Y.; Wang, K. miR-205 suppresses cell migration, invasion and EMT of colon cancer by targeting mouse double minute 4. Mol. Med. Rep. 2020, 22, 633–642.
- Chen, Q.; Zhou, L.; Ye, X.; Tao, M.; Wu, J. miR-145-5p suppresses proliferation, metastasis and EMT of colorectal cancer by targeting CDCA3. Pathol. Res. Pr. 2020, 216, 152872.
- Mansoori, B.; Mohammadi, A.; Naghizadeh, S.; Gjerstorff, M.; Shanehbandi, D.; Shirjang, S.; Najafi, S.; Holmskov, U.; Khaze, V.; Duijf, P.H.; et al. miR-330 suppresses EMT and induces apoptosis by downregulating HMGA2 in human colorectal cancer. J. Cell. Physiol. 2020, 235, 920–931.
- Lin, L.J.; Wang, D.X.; Qu, S.X.; Zhao, H.; Lin, Y. miR-370-3p Alleviates Ulcerative Colitis-Related Colorectal Cancer in Mice Through Inhibiting the Inflammatory Response and Epitheliale-Mesenchymal Transition. Drug Des. Dev. Ther. 2020, 14, 1127–1141.
- Wang, L.Q.; Jiang, F.Q.; Ma, F.; Zhang, B. MiR-873-5p suppresses cell proliferation and epithelial-mesenchymal transition via directly targeting Jumonji domain-containing protein 8 through the NF-kappa B pathway in colorectal cancer. J. Cell Commun. Signal. 2019, 13, 549–560.
- Chang, S.; Sun, G.; Zhang, D.; Li, Q.; Qian, H. MiR-3622a-3p acts as a tumor suppressor in colorectal cancer by reducing stemness features and EMT through targeting spalt-like transcription factor 4. Cell Death Dis. 2020, 11, 1–19.
- Shang, J.-C.; Yu, G.-Z.; Ji, Z.-W.; Wang, X.-Q.; Xia, L. MiR-105 inhibits gastric cancer cells metastasis, epithelial-mesenchymal transition by targeting SOX9. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6160–6169.
- Wang, S.; Chen, Y.; Yu, X.; Lu, Y.; Wang, H.; Wu, F.; Teng, L. miR-129-5p attenuates cell proliferation and epithelial mesenchymal transition via HMGB1 in gastric cancer. Pathol. Res. Pract. 2019, 215, 676–682.
- Li, J.Y.; Zhang, B.; Cui, J.Z.; Liang, Z.; Liu, K.X. miR-203 Inhibits the Invasion and EMT of Gastric Cancer Cells by Directly Targeting Annexin A4. Oncol. Res. 2019, 27, 789–799.
- Xu, J.P.; You, Q.; Wei, Z.R.; Fu, H.P.; Zhang, Y.; Hu, Z.Q.; Cai, Q.P. miR-519 inhibits epithelial-mesenchymal transition and biologic behavior of gastric cancer cells by down-regulating FOXQ1. Int. J. Clin. Exp. Pathol. 2020, 13, 425–436.
- Li, D.; Tian, B.; Jin, X.S. miR-630 Inhibits Epithelial-to-Mesenchymal Transition (EMT) by Regulating the Wnt/beta-Catenin Pathway in Gastric Cancer Cells. Oncol. Res. 2019, 27, 9–17.
- Wu, K.Z.; Zhang, C.D.; Zhang, C.; Pei, J.P.; Dai, D.Q. miR-665 Suppresses the Epithelial-Mesenchymal Transition and Progression of Gastric Cancer by Targeting CRIM1. Cancer Manag. Res. 2020, 12, 3489–3501.
- Ostrom, Q.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16, iv1–iv63.
- Feng, S.Y.; Yao, J.; Zhang, Z.B.; Zhang, Y.Y.; Zhang, Z.Y.; Liu, J.L.; Tan, W.L.; Sun, C.H.; Chen, L.; Yu, X.G. miR-96 inhibits EMT by targeting AEG-1 in glioblastoma cancer cells. Mol. Med. Rep. 2018, 17, 2964–2972.
- Dai, B.; Zhou, G.; Hu, Z.; Zhu, G.; Mao, B.; Su, H.; Jia, Q. MiR-205 suppresses epithelial–mesenchymal transition and inhibits tumor growth of human glioma through down-regulation of HOXD9. Biosci. Rep. 2019, 39.
- Li, Z.; Qian, R.; Zhang, J.; Shi, X. MiR-218-5p targets LHFPL3 to regulate proliferation, migration, and epithelial–mesenchymal transitions of human glioma cells. Biosci. Rep. 2019, 39.
- Huang, W.; Shi, Y.; Han, B.; Wang, Q.L.; Zhang, B.; Qi, C.J.; Liu, F. miR-802 inhibits the proliferation, invasion, and epithelial-mesenchymal transition of glioblastoma multiforme cells by directly targeting SIX4. Cell Biochem. Funct. 2020, 38, 66–76.
- Ma, B.Y.; Xu, J.; Chen, G.; Wei, D.; Gu, P.Y.; Li, L.X.; Hu, W.X. miR-876-5p exerts tumor suppressor function by targeting TWIST1 and regulating the epithelial-mesenchymal transition in glioblastoma. Int. J. Clin. Exp. Med. 2020, 13, 1454–1463.
- Zhu, D.; Gu, L.; Li, Z.; Jin, W.; Lu, Q.; Ren, T. MiR-138-5p suppresses lung adenocarcinoma cell epithelial-mesenchymal transition, proliferation and metastasis by targeting ZEB2. Pathol. Res. Pr. 2019, 215, 861–872.
- Liu, Q.; Chen, J.; Wang, B.; Zheng, Y.; Wan, Y.; Wang, Y.; Zhou, L.; Liu, S.; Li, G.; Yan, Y. miR-145 modulates epithelial-mesenchymal transition and invasion by targeting ZEB2 in non–small cell lung cancer cell lines. J. Cell. Biochem. 2019, 120, 8409–8418.
- Han, Q.; Cheng, P.; Yang, H.J.; Liang, H.P.; Lin, F.C. miR-146b Reverses epithelial-mesenchymal transition via targeting PTP1B in cisplatin-resistance human lung adenocarcinoma cells. J. Cell. Biochem. 2020, 121, 3901–3912.
- Du, W.W.; Tang, H.C.; Lei, Z.; Zhu, J.J.; Zeng, Y.Y.; Liu, Z.Y.; Huang, J.A. miR-335-5p inhibits TGF-beta 1-induced epithelial-mesenchymal transition in non-small cell lung cancer via ROCK1. Respir. Res. 2019, 20, 11.
- Chang, J.; Gao, F.; Chu, H.; Lou, L.; Wang, H.; Chen, Y. miR-363-3p inhibits migration, invasion, and epithelial-mesenchymal transition by targeting NEDD9 and SOX4 in non-small-cell lung cancer. J. Cell. Physiol. 2020, 235, 1808–1820.
- Liu, B.; Wang, Z.; Cheng, S.; Du, L.; Yin, Y.; Yang, Z.; Zhou, J. miR-379 inhibits cell proliferation and epithelial-mesenchymal transition by targeting CHUK through the NF-κB pathway in non-small cell lung cancer. Mol. Med. Rep. 2019, 20, 1418–1428.
- Wang, S.H.; Wu, Y.Y.; Yang, S.H.; Liu, X.C.; Lu, Y.; Liu, F.X.; Li, G.X.; Tian, G.R. miR-874 directly targets AQP3 to inhibit cell proliferation, mobility and EMT in non-small cell lung cancer. Thorac. Cancer 2020, 11, 1550–1558.
- Jiang, K.Q.; Zhao, T.; Shen, M.J.; Zhang, F.Q.; Duan, S.Z.; Lei, Z.; Chen, Y.B. MiR-940 inhibits TGF-beta-induced epithelial-mesenchymal transition and cell invasion by targeting Snail in non-small cell lung cancer (vol 10, pg 2735, 2019). J. Cancer 2020, 11, 4897–4898.
- An, N.; Zheng, B. MiR-203a-3p Inhibits Pancreatic Cancer Cell Proliferation, EMT, and Apoptosis by Regulating SLUG. Technol. Cancer Res. Treat. 2020, 19.
- Sun, J.; Chen, L.; Dong, M. MiR-338-5p Inhibits EGF-Induced EMT in Pancreatic Cancer Cells by Targeting EGFR/ERK Signaling. Front. Oncol. 2021, 11, 11.
- Zhang, W.; Ji, W.; Li, T.; Liu, T.; Zhao, X. MiR-145 functions as a tumor suppressor in Papillary Thyroid Cancer by inhibiting RAB5C. Int. J. Med. Sci. 2020, 17, 1992–2001.
- Fan, X.; Zhao, Y. miR-451a inhibits cancer growth, epithelial-mesenchymal transition and induces apoptosis in papillary thyroid cancer by targeting PSMB8. J. Cell. Mol. Med. 2019, 23, 8067–8075.
- Pan, X.-M.; He, X.-Y.; Yang, Y.-L.; Jia, W.-J.; Yang, Z.-Q.; Yan, D.; Ma, J.-X. MiR-630 inhibits papillary thyroid carcinoma cell growth, metastasis, and epithelial-mesenchymal transition by suppressing JAK2/STAT3 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2453–2460.
- Gu, X.B.; Dong, M.L.; Liu, Z.Y.; Yang, J.; Shi, Y.G. MiR-499a-5p Inhibits Proliferation, Invasion, Migration, and Epithelial-Mesenchymal Transition, and Enhances Radiosensitivity of Cervical Cancer Cells via Targeting eIF4E. Oncotarget Ther. 2020, 13, 2913–2924.
- Lu, Y.J.; Li, X.R.; Zuo, Y.Z.; Xu, Q.; Liu, L.; Wu, H.Y.; Chen, L.; Zhang, Y.; Liu, Y.; Li, Y.H. miR-373-3p inhibits epithelial-mesenchymal transition via regulation of TGF beta R2 in choriocarcinoma. J. Obstet. Gynaecol. Res. 2021, 47, 2417–2432.
- Pan, H.; Hong, Y.; Yu, B.; Li, L.; Zhang, X. miR-4429 Inhibits Tumor Progression and Epithelial-Mesenchymal Transition Via Targeting CDK6 in Clear Cell Renal Cell Carcinoma. Cancer Biotherapy Radiopharm. 2019, 34, 334–341.
- Wang, X.; Zhao, Y.; Lu, Q.; Fei, X.; Lu, C.; Li, C.; Chen, H. MiR-34a-5p Inhibits Proliferation, Migration, Invasion and Epithelial-mesenchymal Transition in Esophageal Squamous Cell Carcinoma by Targeting LEF1 and Inactivation of the Hippo-YAP1/TAZ Signaling Pathway. J. Cancer 2020, 11, 3072–3081.
- Wu, H.; Liu, J.; Zhang, Y.; Li, Q.; Wang, Q.; Gu, Z. miR-22 suppresses cell viability and EMT of ovarian cancer cells via NLRP3 and inhibits PI3K/AKT signaling pathway. Clin. Transl. Oncol. 2021, 23, 257–264.
- Chung, V.Y.; Tan, T.Z.; Tan, M.; Wong, M.K.; Kuay, K.T.; Yang, Z.; Ye, J.R.; Muller, J.; Koh, C.M.; Guccione, E.; et al. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci. Rep. 2016, 6, 15.
- Abdeyrim, A.; Cheng, X.; Lian, M.; Tan, Y. miR-490-5p regulates the proliferation, migration, invasion and epithelial-mesenchymal transition of pharyngolaryngeal cancer cells by targeting mitogen-activated protein kinase kinasekinase 9. Int. J. Mol. Med. 2019, 44, 240–252.
- Ma, T.; Zhao, Z.G.; Wang, Z.M.; Wang, C.N.; Zhang, L.P. MiR-940 inhibits migration and invasion of tongue squamous cell carcinoma via regulating CXCR2/NF-kappa B system-mediated epithelial-mesenchymal transition. Naunyn-Schmiedebergs Arch. Pharmacol. 2019, 392, 1359–1369.




