
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cesare Gagliardo | + 2260 word(s) | 2260 | 2021-07-12 06:04:10 | | | |
| 2 | Vivi Li | Meta information modification | 2260 | 2021-07-21 03:42:17 | | | | |
| 3 | Paola Feraco | Meta information modification | 2260 | 2021-07-21 06:58:39 | | | | |
| 4 | Jamir Pitton Rissardo | + 38 word(s) | 2298 | 2023-09-07 01:57:10 | | |
Video Upload Options
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, characterized by motor and non-motor symptoms due to the degeneration of the pars compacta of the substantia nigra (SNc) with dopaminergic denervation of the striatum. Although the diagnosis of PD is principally based on a clinical assessment, great efforts have been expended over the past two decades to evaluate reliable biomarkers for PD. Among these biomarkers, magnetic resonance imaging (MRI)-based biomarkers may play a key role. Conventional MRI sequences are considered by many in the field to have low sensitivity, while advanced pulse sequences and ultra-high-field MRI techniques have brought many advantages, particularly regarding the study of brainstem and subcortical structures. Nowadays, nigrosome imaging, neuromelanine-sensitive sequences, iron-sensitive sequences, and advanced diffusion weighted imaging techniques afford new insights to the non-invasive study of the SNc.
1. Introduction
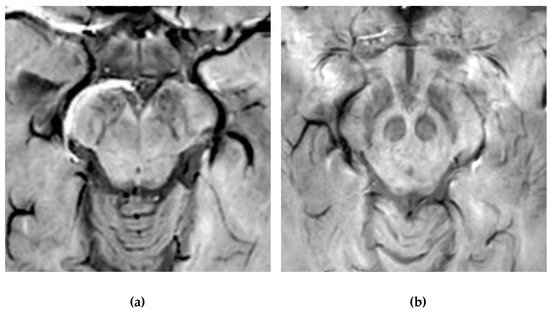
2. Neuromelanin Imaging
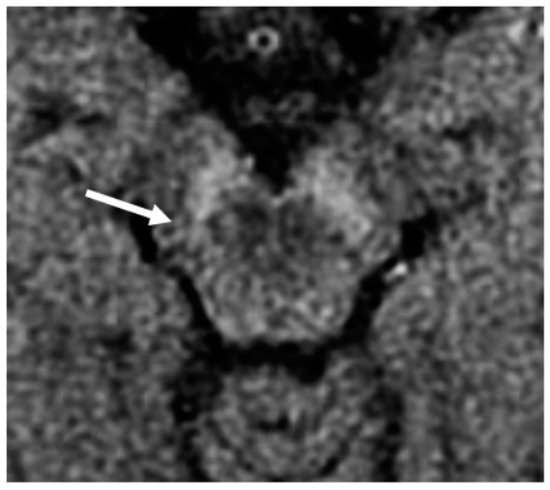
3. Nigrosome-1 Imaging
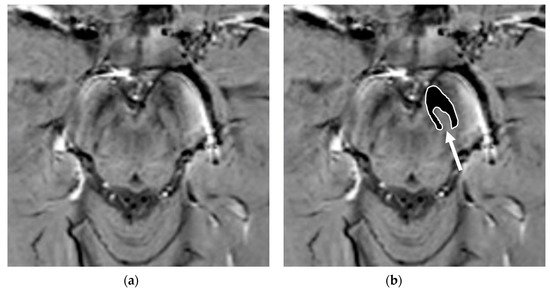
It is important to note that adequate visualization of the swallow tail sign requires targeted 3D high-resolution SWI. In the original publication, the parameters were: FEEPI, TR/TE 60/30, echo train length 5, flip angle 19°; 70 slices; voxel size 0.55 x 0.55 x 0.7 mm.[53]
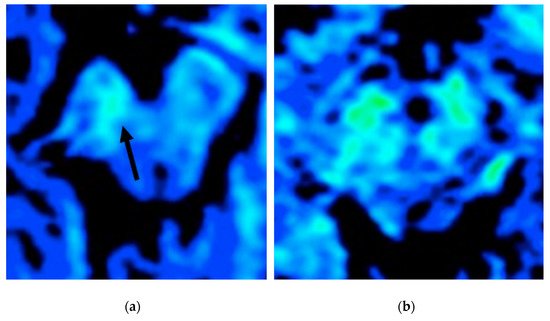
4. Iron Imaging
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912.
- Dickson, D.W. Neuropathology of Parkinson disease. Park. Relat. Disord. 2018, 46, S30–S33.
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551.
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134.
- Postuma, R.B.; Berg, D.; Stern, M.B.; Poewe, W.; Olanow, C.W.; Oertel, W.H.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601.
- Schapira, A.H.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450.
- Monastero, R.; Cicero, C.E.; Baschi, R.; Davì, M.; Luca, A.; Restivo, V.; Zangara, C.; Fierro, B.; Zappia, M.; Nicoletti, A. Mild cognitive impairment in Parkinson’s disease: The Parkinson’s disease cognitive study (PACOS). J. Neurol. 2018, 265, 1050–1058.
- Nicoletti, A.; Luca, A.; Baschi, R.; Cicero, C.E.; Mostile, G.; Davì, M.; Pilati, L.; Restivo, V.; Zappia, M.; Monastero, R. Incidence of Mild Cognitive Impairment and Dementia in Parkinson’s Disease: The Parkinson’s Disease Cognitive Impairment Study. Front. Aging Neurosci. 2019, 11, 21.
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231.
- Chen-Plotkin, A.S.; Albin, R.; Alcalay, R.; Babcock, D.; Bajaj, V.; Bowman, D.; Buko, A.; Cedarbaum, J.; Chelsky, D.; Cookson, M.R.; et al. Finding useful biomarkers for Parkinson’s disease. Sci. Transl. Med. 2018, 10, eaam6003.
- Heim, B.; Krismer, F.; De Marzi, R.; Seppi, K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural Transm. 2017, 124, 915–964.
- Zucca, F.A.; Basso, E.; Cupaioli, F.A.; Ferrari, E.; Sulzer, D.; Casella, L.; Zecca, L. Neuromelanin of the Human Substantia Nigra: An Update. Neurotox. Res. 2014, 25, 13–23.
- Trujillo, P.; Summers, P.; Ferrari, E.; Zucca, F.A.; Sturini, M.; Mainardi, L.T.; Cerutti, S.; Smith, A.K.; Smith, S.A.; Zecca, L.; et al. Contrast mechanisms associated with neuromelanin-MRI. Magn. Reson. Med. 2017, 78, 1790–1800.
- Sasaki, M.; Shibata, E.; Kudo, K.; Tohyama, K. Neuromelanin-sensitive MRI basics, technique, and clinical applications. Clin. Neuroradiol. 2008, 18, 147–153.
- Pluijm, M.; Cassidy, C.; Ms, M.Z.; Wallert, E.; Bruin, K.; Booij, J.; Haan, L.; Horga, G.; Giessen, E. Reliability and Reproducibility of Neuromelanin-Sensitive Imaging of the Substantia Nigra: A Comparison of Three Different Sequences. J. Magn. Reson. Imaging 2021, 53, 712–721.
- Kitao, S.; Matsusue, E.; Fujii, S.; Miyoshi, F.; Kaminou, T.; Kato, S.; Ito, H.; Ogawa, T. Correlation between pathology and neuromelanin MR imaging in Parkinson’s disease and dementia with Lewy bodies. Neuroradiology 2013, 55, 947–953.
- Reimão, S.; Lobo, P.P.; Neutel, D.; Guedes, L.C.; Coelho, M.; Rosa, M.M.; Azevedo, P.; Ferreira, J.; Abreu, D.; Gonçalves, N.; et al. Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson’s disease. Mov. Disord. 2015, 30, 953–959.
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.J.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Park. Dis. 2018, 4, 1–13.
- Matsuura, K.; Maeda, M.; Tabei, K.-I.; Umino, M.; Kajikawa, H.; Satoh, M.; Kida, H.; Tomimoto, H. A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease. Neurosci. Lett. 2016, 633, 112–117.
- Takahashi, H.; Watanabe, Y.; Tanaka, H.; Mihara, M.; Mochizuki, H.; Yamamoto, K.; Liu, T.; Wang, Y.; Tomiyama, N. Comprehensive MRI quantification of the substantia nigra pars compacta in Parkinson’s disease. Eur. J. Radiol. 2018, 109, 48–56.
- Takahashi, H.; Watanabe, Y.; Tanaka, H.; Mihara, M.; Mochizuki, H.; Liu, T.; Wang, Y.; Tomiyama, N. Quantifying changes in nigrosomes using quantitative susceptibility mapping and neuromelanin imaging for the diagnosis of early-stage Parkinson’s disease. Br. J. Radiol. 2018, 91, 20180037.
- Prasad, S.; Stezin, A.; Lenka, A.; George, L.; Saini, J.; Yadav, R.; Pal, P.K. Three-dimensional neuromelanin-sensitive magnetic resonance imaging of the substantia nigra in Parkinson’s disease. Eur. J. Neurol. 2018, 25, 680–686.
- Pyatigorskaya, N.; Magnin, B.; Mongin, M.; Yahia-Cherif, L.; Valabregue, R.; Arnaldi, D.; Ewenczyk, C.; Poupon, C.; Vidailhet, M.; Lehéricy, S. Comparative Study of MRI Biomarkers in the Substantia Nigra to Discriminate Idiopathic Parkinson Disease. Am. J. Neuroradiol. 2018, 39, 1460–1467.
- Biondetti, E.; Gaurav, R.; Yahia-Cherif, L.; Mangone, G.; Pyatigorskaya, N.; Valabrègue, R.; Ewenczyk, C.; Hutchison, M.; François, C.; Arnulf, I.; et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain 2020, 143, 2757–2770.
- Wang, J.; Li, Y.; Huang, Z.; Wan, W.; Zhang, Y.; Wang, C.; Cheng, X.; Ye, F.; Liu, K.; Fei, G.; et al. Neuromelanin-sensitive magnetic resonance imaging fea-tures of the substantia nigra and locus coeruleus in de novo Parkinson’s disease and its phenotypes. Eur. J. Neurol. 2018, 25, 949-e73.
- Xiang, Y.; Gong, T.; Wu, J.; Li, J.; Chen, Y.; Wang, Y.; Li, S.; Cong, L.; Lin, Y.; Han, Y.; et al. Subtypes evaluation of motor dysfunction in Parkinson’s disease using neuromelanin-sensitive magnetic resonance imaging. Neurosci. Lett. 2017, 638, 145–150.
- Wang, J.; Huang, Z.; Li, Y.; Ye, F.; Wang, C.; Zhang, Y.; Cheng, X.; Fei, G.; Liu, K.; Zeng, M.; et al. Neuromelanin-sensitive MRI of the substantia nigra: An im-aging biomarker to differentiate essential tremor from tremor-dominant Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 58, 3–8.
- Chougar, L.; Pyatigorskaya, N.; Degos, B.; Grabli, D.; Lehéricy, S. The Role of Magnetic Resonance Imaging for the Diagno-sis of Atypical Parkinsonism. Front. Neurol. 2020, 11, 665.
- Pyatigorskaya, N.; Gaurav, R.; Arnaldi, D.; Leu-Semenescu, S.; Yahia-Cherif, L.; Valabregue, R.; Vidailhet, M.; Arnulf, I.; Lehéricy, S. Magnetic Resonance Imaging Biomarkers to Assess Substantia Nigra Damage in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Sleep 2017, 40.
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain 1999, 122, 1421–1436.
- Schwarz, S.T.; Mougin, O.; Xing, Y.; Blazejewska, A.; Bajaj, N.; Auer, D.P.; Gowland, P. Parkinson’s disease related signal change in the nigrosomes 1–5 and the substantia nigra using T2* weighted 7T MRI. NeuroImage Clin. 2018, 19, 683–689.
- Lehéricy, S.; Bardinet, E.; Poupon, C.; Vidailhet, M.; François, C. 7 tesla magnetic resonance imaging: A closer look at sub-stantia nigra anatomy in Parkinson’s disease. Mov Disord 2014, 29, 1574–1581.
- Schwarz, S.T.; Afzal, M.; Morgan, P.S.; Bajaj, N.; Gowland, P.A.; Auer, D.P. The “swallow tail” appearance of the healthy nigro-some—A new accurate test of Parkinson’s disease: A case-control and retrospective cross-sectional MRI study at 3T. PLoS ONE 2014, 9, 93814.
- Gao, P.; Zhou, P.-Y.; Wang, P.-Q.; Zhang, G.-B.; Liu, J.-Z.; Xu, F.; Yang, F.; Wu, X.-X.; Li, G. Universality analysis of the existence of substantia nigra “swallow tail” appearance of non-Parkinson patients in 3T SWI. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1307–1314.
- Cheng, Z.; He, N.; Huang, P.; Li, Y.; Tang, R.; Sethi, S.K.; Ghassaban, K.; Yerramsetty, K.K.; Palutla, V.K.; Chen, S.; et al. Imaging the Nigrosome 1 in the substantia nigra using sus-ceptibility weighted imaging and quantitative susceptibility mapping: An application to Parkinson’s disease. Neuroimage Clin. 2020, 25, 102–103.
- Schmidt, M.A.; Engelhorn, T.; Marxreiter, F.; Winkler, J.; Lang, S.; Kloska, S.; Goelitz, P.; Doerfler, A. Ultra high-field SWI of the substantia nigra at 7T: Reliability and consistency of the swallow-tail sign. BMC Neurol. 2017, 17, 1–6.
- Kau, T.; Hametner, S.; Endmayr, V.; Deistung, A.; Prihoda, M.; Haimburger, E.; Menard, C.; Haider, T.; Höftberger, R.; Robinson, S.; et al. Microvessels may Confound the “Swallow Tail Sign” in Normal Aged Midbrains: A Postmortem 7 T SW-MRI Study. J. Neuroimaging 2018, 29, 65–69.
- Gao, P.; Zhou, P.-Y.; Li, G.; Zhang, G.-B.; Wang, P.-Q.; Liu, J.-Z.; Xu, F.; Yang, F.; Wu, X.-X. Visualization of nigrosomes-1 in 3T MR susceptibility weighted imaging and its absence in diagnosing Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4603–4609.
- Mahlknecht, P.; Krismer, F.; Poewe, W.; Seppi, K. Meta-analysis of dorsolateral nigral hyperintensity on magnetic reso-nance imaging as a marker for Parkinson’s disease. Mov. Disord. 2017, 32, 619–623.
- Sung, Y.H.; Lee, J.; Nam, Y.; Shin, H.-G.; Noh, Y.; Shin, D.H.; Kim, E.Y. Differential involvement of nigral subregions in idiopathic parkinson’s disease. Hum. Brain Mapp. 2018, 39, 542–553.
- Akly, M.S.P.; Stefani, C.V.; Ciancaglini, L.; Bestoso, J.S.; Funes, J.A.; Bauso, D.J.; Besada, C.H. Accuracy of nigrosome-1 detection to discriminate patients with Parkinson’s disease and essential tremor. Neuroradiol. J. 2019, 32, 395–400.
- Calloni, S.F.; Conte, G.; Sbaraini, S.; Cilia, R.; Contarino, V.E.; Avignone, S.; Sacilotto, G.; Pezzoli, G.; Triulzi, F.M.; Scola, E. Multiparametric MR imaging of Parkin-sonisms at 3 tesla: Its role in the differentiation of idiopathic Parkinson’s disease versus atypical Parkinsonian disorders. Eur. J. Radiol. 2018, 109, 95–100.
- Stezin, A.; Naduthota, R.M.; Botta, R.; Varadharajan, S.; Lenka, A.; Saini, J.; Yadav, R.; Pal, P.K. Clinical utility of visualisation of nigro-some-1 in patients with Parkinson’s disease. Eur. Radiol. 2018, 28, 718–726.
- Sung, Y.H.; Noh, Y.; Lee, J.; Kim, E.Y. Drug-induced Parkinsonism versus Idiopathic Parkinson Disease: Utility of Nigro-some 1 with 3-T Imaging. Radiology 2016, 279, 849–858.
- De Marzi, R.; Seppi, K.; Högl, B.; Müller, C.; Scherfler, C.; Stefani, A.; Iranzo, A.; Tolosa, E.; Santamarìa, J.; Gizewski, E.R.; et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann. Neurol. 2016, 79, 1026–1030.
- Rizzo, G.; De Blasi, R.; Capozzo, R.; Tortelli, R.; Barulli, M.R.; Liguori, R.; Grasso, D.; Logroscino, G. Loss of Swallow Tail Sign on Susceptibil-ity-Weighted Imaging in Dementia with Lewy Bodies. J. Alzheimers Dis. 2019, 67, 61–65.
- Kathuria, H.; Mehta, S.; Ahuja, C.K.; Chakravarty, K.; Ray, S.; Mittal, B.R.; Singh, P.; Lal, V. Utility of Imaging of Nigrosome-1 on 3T MRI and Its Comparison with 18F-DOPA PET in the Diagnosis of Idiopathic Parkinson Disease and Atypical Parkinsonism. Mov. Disord. Clin. Pr. 2021, 8, 224–230.
- Kim, J.-M.; Jeong, H.-J.; Bae, Y.J.; Park, S.-Y.; Kim, E.; Kang, S.Y.; Oh, E.S.; Kim, K.J.; Jeon, B.; Kim, S.E.; et al. Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Park. Relat. Disord. 2016, 26, 47–54.
- Frosini, D.; Ceravolo, R.; Tosetti, M.; Bonuccelli, U.; Cosottini, M. Nigral involvement in atypical parkinsonisms: Evidence from a pilot study with ultra-high field MRI. J. Neural Transm. 2016, 123, 509–513.
- Oh, S.W.; Shin, N.Y.; Lee, J.J.; Lee, S.K.; Lee, P.H.; Lim, S.M.; Kim, J.W. Correlation of 3D FLAIR and Dopamine Transporter Im-aging in Patients With Parkinsonism. AJR Am. J. Roentgenol. 2016, 207, 1089–1094.
- Simões, R.M.; Caldas, A.C.; Grilo, J.; Correia, D.; Guerreiro, C.; Lobo, P.P.; Valadas, A.; Fabbri, M.; Guedes, L.C.; Coelho, M.; et al. A distinct neuromelanin magnetic resonance imaging pattern in parkinsonian multiple system atrophy. BMC Neurol. 2020, 20, 1–12.
- Vitali, P.; Pan, M.I.; Palesi, F.; Germani, G.; Faggioli, A.; Anzalone, N.; Francaviglia, P.; Minafra, B.; Zangaglia, R.; Pacchetti, C.; et al. Substantia Nigra Volumetry with 3-T MRI in De Novo and Advanced Parkinson Disease. Radiology 2020, 296, 401–410.
- Malte Brammerloh; Evgeniya Kirilina; Anneke Alkemade; Pierre-Louis Bazin; Caroline Jantzen; Carsten Jäger; Andreas Herrler; Kerrin J. Pine; Penny A. Gowland; Markus Morawski; et al.Birte U. ForstmannNikolaus Weiskopf Swallow Tail Sign: Revisited. Radiol. 2022, 305, 674-677.
- Wang, J.Y.; Zhuang, Q.Q.; Zhu, L.B.; Zhu, H.; Li, T.; Li, R.; Chen, S.F.; Huang, C.P.; Zhang, X.; Zhu, J.H. Meta-analysis of brain iron levels of Parkinson’s disease pa-tients determined by postmortem and MRI measurements. Sci. Rep. 2016, 6, 36669.
- Wang, Y.; Butros, S.R.; Shuai, X.; Dai, Y.; Chen, C.; Liu, M.; Haacke, E.M.; Hu, J.; Xu, H. Different iron-deposition patterns of multiple system atro-phy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibil-ity-weighted imaging. AJNR Am. J. Neuroradiol. 2012, 33, 266–273.
- Hopes, L.; Grolez, G.; Moreau, C.; Lopes, R.; Ryckewaert, G.; Carrière, N.; Auger, F.; Laloux, C.; Petrault, M.; Devedjian, J.-C.; et al. Magnetic Resonance Imaging Features of the Nigrostriatal System: Biomarkers of Parkinson’s Disease Stages? PLoS ONE 2016, 11, e0147947.
- Du, G.; Lewis, M.M.; Sica, C.; He, L.; Connor, J.R.; Kong, L.; Mailman, R.B.; Huang, X. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov. Disord. 2018, 33, 1423–1431.
- Wieler, M.; Gee, M.; Camicioli, R.; Martin, W.W. Freezing of gait in early Parkinson’s disease: Nigral iron content estimated from magnetic resonance imaging. J. Neurol. Sci. 2016, 361, 87–91.
- Du, G.; Liu, T.; Lewis, M.M.; Kong, L.; Wang, Y.; Connor, J.; Mailman, R.B.; Huang, X. Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Mov. Disord. 2016, 31, 317–324.
- Azuma, M.; Hirai, T.; Yamada, K.; Yamashita, S.; Ando, Y.; Tateishi, M.; Iryo, Y.; Yoneda, T.; Kitajima, M.; Wang, Y.; et al. Lateral asymmetry and spatial difference of iron deposition in the substantia nigra of patients with Parkinson disease measured with quantitative susceptibility map-ping. AJNR Am. J. Neuroradiol. 2016, 37, 782–788.
- Langkammer, C.; Pirpamer, L.; Seiler, S.; Deistung, A.; Schweser, F.; Franthal, S.; Homayoon, N.; Katschnig-Winter, P.; Koegl-Wallner, M.; Pendl, T.; et al. Quantitative Susceptibility Mapping in Parkinson’s Disease. PLoS ONE 2016, 11, e0162460.
- Sun, J.; Lai, Z.; Ma, J.; Gao, L.; Chen, M.; Chen, J.; Fang, J.; Fan, Y.; Bao, Y.; Zhang, D.; et al. Quantitative Evaluation of Iron Content in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2020, 35, 478–485.
- Saikiran, P.; Priyanka. Effectiveness of QSM over R2* in assessment of parkinson’s disease—A systematic review. Neurol. India. 2020, 68, 278–281.
- An, H.; Zeng, X.; Niu, T.; Li, G.; Yang, J.; Zheng, L.; Zhou, W.; Liu, H.; Zhang, M.; Huang, D.; et al. Quantifying iron deposition within the substantia nigra of Parkin-son’s disease by quantitative susceptibility mapping. J. Neurol. Sci. 2018, 386, 46–52.
- Fedeli, M.P.; Contarino, V.E.; Siggillino, S.; Samoylova, N.; Calloni, S.; Melazzini, L.; Conte, G.; Sacilotto, G.; Pezzoli, G.; Triulzi, F.M.; et al. Iron deposition in Parkinsonisms: A Quantitative Susceptibility Mapping study in the deep grey matter. Eur. J. Radiol. 2020, 133, 109394.
- Bergsland, N.; Zivadinov, R.; Schweser, F.; Hagemeier, J.; Lichter, D.; Guttuso, T., Jr. Ventral posterior substantia nigra iron increases over 3 years in Parkinson’s disease. Mov. Disord. 2019, 34, 1006–1013.
- Guan, X.; Xuan, M.; Gu, Q.; Huang, P.; Liu, C.; Wang, N.; Xu, X.; Luo, W.; Zhang, M. Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed. 2017, 30, e3489.
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopa-mine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448.
- Mazzucchi, S.; Frosini, D.; Costagli, M.; Del Prete, E.; Donatelli, G.; Cecchi, P.; Migaleddu, G.; Bonuccelli, U.; Ceravolo, R.; Cosottini, M. Quantitative susceptibility mapping in atypical Parkinsonisms. NeuroImage Clin. 2019, 24, 101999.
- Sjöström, H.; Granberg, T.; Westman, E.; Svenningsson, P. Quantitative susceptibility mapping differentiates between par-kinsonian disorders. Parkinsonism Relat. Disord. 2017, 44, 51–57.
- Lewis, M.M.; Du, G.; Baccon, J.; Snyder, A.M.; Murie, B.; Cooper, F.; Stetter, C.; Kong, L.; Sica, C.; Mailman, R.B.; et al. Susceptibility MRI captures nigral pathology in pa-tients with parkinsonian syndromes. Mov. Disord. 2018, 33, 1432–1439.




