2. Neuromelanin Imaging
Neuromelanin (NM) is a black pigment that is composed of melanin, proteins, lipids, and metal ions, and it is found in the SNc (in the nigral matrix and the nigrosomes). NM plays a protective role against the accumulation of toxic catecholamine derivatives and oxidative stress
[12][19]. NM normally accumulates during aging but is strongly reduced in patients with PD as a result of the selective loss of dopaminergic neurons containing NM. The latter has a paramagnetic T1 reduction effect on MRI due to the presence of melanin-iron complexes
[13][20]. With high-resolution turbo spin echo (TSE) T1W images with a magnetization transfer (MT) pulse, it is possible to suppress brain tissue signals due to the prolongation of the T1 relaxation time
[14][21]. Hence, nuclei-containing NM can be visualized as a separate hyperintense area relative to the surrounding hypointense brain tissue. Although the use of TSE T1W images has been consistently applied to visualizing NM, the gradient recalled echo (GRE) sequence with MT pulse has recently been demonstrated to achieve the sharpest contrast and lowest variability when compared with a T1W TSE-MT sequence
[15][22].
NM-MRI is a validated technique with which to quantify the loss of dopaminergic neurons in the SN of patients with PD. The loss of SN hyperintensity in the T1W-MT sequence is associated with the loss of neuromelanin-containing neurons in PD and DLB, as confirmed in post-mortem studies
[16][23]. Indeed, patients suffering from PD have significantly reduced NM signal in the SN (
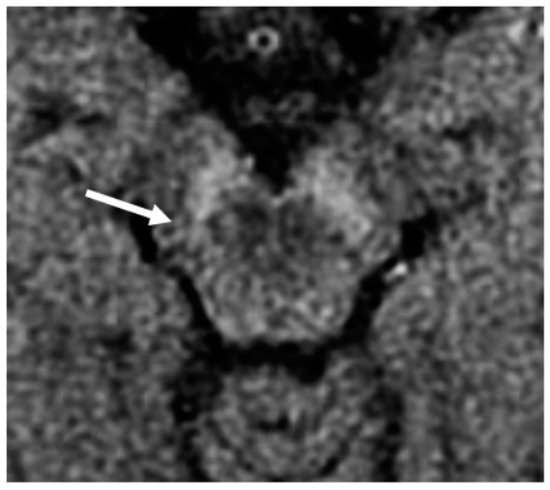
Figure 2), which invariably decreases on follow-up
[17][18][19][20][21][22][24,25,26,27,28,29].
Figure 2. NM-MRI sequence with an explicit MT preparation pulse, scanned with a 1.5T MR scanner at the level of the SN in a PD patient with asymmetrical motor symptoms onset: the loss of hyperintensity in the posterolateral aspect of the right SN (arrow) correlated well with the clinical presentation.
Measuring NM-sensitive images correlates with elevated diagnostic accuracy for PD: the sensitivity and specificity of this technique to distinguish between PD and control patients are 88% and 80%, respectively
[23][30]. The NM signal changes commence in the posterolateral motor areas of the SN, and then proceed to the medial areas
[24][31]. Hence, the evaluation of longitudinal changes in the NM signal in PD patients could be used as a marker indicating disease progression. A reduction in NM signal has been reported to be not specific for motor or non-motor PD subtypes
[25][32]. On the other hand, a potential diagnostic value of NM-MRI in discriminating PD motor phenotypes has been proposed
[26][33]. Indeed, patients with postural instability gait difficulty phenotype display increased severe signal attenuation in the medial part of the SNc, in comparison with tremor-dominant PD patients
[26][33]. Furthermore, the use of NM-MRI-based imaging is capable of differentiating between untreated essential tremor (ET) and de novo PD with a tremor-dominant phenotype
[27][34]. Finally, a NM signal decrease has been observed in patients suffering from idiopathic rapid eye movement sleep behavior disorder, which is considered a prodromal phase of Parkinsonism and PD
[28][29][35,36].
3. Nigrosome-1 Imaging
Nigrosomes are dopaminergic neurons within the SNc that are characterized by high NM levels and a paucity of iron. They can be subdivided into five different regions (nigrosome 1 to 5), the largest of which, nigrosome-1 (located in the dorsolateral part of SNc
[30][12]), has been shown to play a key role in the neuropathology of PD. Indeed, the greatest loss of dopaminergic neurons in PD patients occurs in the nigrosome-1. It was first detected in vivo by 7.0-Tesla (7T) MRI as a hyperintense, ovoid area on T2*-weighted images, within the dorsolateral border of the hypointense SN pars compacta
[31][32][37,38]. Similar findings can be found with the more commonly used 3-Tesla (3T) MRI
[33][39]. By using T2* or susceptibility-weighted imaging (SWI), researchers have also termed this region
dorsolateral nigral hyperintensity or a
swallow-tail sign (STS) (
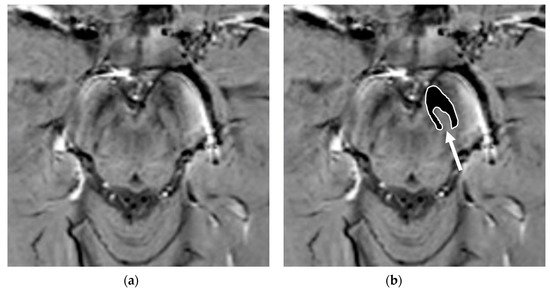
Figure 3).
Figure 3. Susceptibility-weighted imaging (SWI) scan performed with a 3T MR scanner in a normally aging brain of a 65-year-old male who underwent a brain MRI examination for persistent headaches. A raw slice passing through the mesencephalon (a) and the same slice with superimposed highlighted SNc (white surrounded black ROI), thereby demonstrating the normal appearance of the nigrosome-1 (hyperintense area pointed by the white arrow) or swallow-tail sign (b).
Normal nigrosome-1 and the surrounding structure of the dorsolateral SN appear as a swallow tail
[34][40], and they can be visualized in 95% of healthy subjects
[35][36][41,42]. Iron deposits and microvessels have been reported as contributing to the hyposignal surrounding nigrosome-1 in the SWI of normal aged midbrains
[37][43]. Nigrosome-1 in PD patients displays a significant loss of STS on T2* weighted images, probably due to a reduction in NM within dopaminergic neurons, an increase in free iron (which induces local inhomogeneity in the magnetic field resulting in signal loss), or a loss of paramagnetic NM–iron complexes
[38][39][44,45]. As the disease advances, a loss of T2* hyperintensity in PD has been demonstrated to progress from nigrosome-1 to nigrosome-4
[40][46]. The absence of STS may assist in the differential diagnosis for PD if compared with controls and ET, ultimately reaching high sensitivity and specificity
[41][34][42][43][17,40,47,48] (
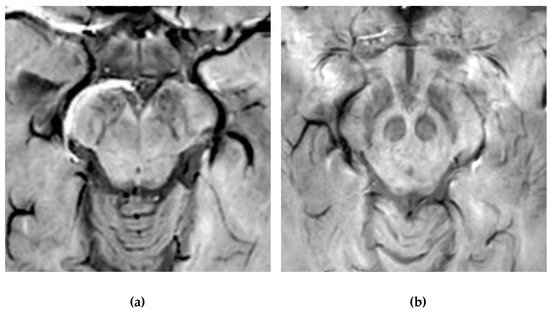
Figure 4).
Figure 4. Susceptibility-weighted imaging (SWI) scan performed with a 3T MR. (a) Presence of regular swallow tail sign in a healthy patient; (b) loss of swallow tail sign in a patient with Parkinson’s disease.
Moreover, the imaging of nigrosome-1 with 3T MR has been demonstrated to differentiate drug-induced Parkinsonism from idiopathic PD with elevated accuracy, thereby being of assistance in screening patients who required dopamine transporter imaging
[44][49]. Furthermore, a loss of STS has also been observed in patients suffering from idiopathic rapid eye movement sleep behavior disorder and DLB
[45][46][50,51]. Whilst the loss of nigrosome-1 on SWI sequences may assist as a potential imaging biomarker in the diagnosis of degenerative parkinsonian syndromes, it cannot differentiate between idiopathic PD and PS
[47][48][52,53]. Nevertheless, it has been reported that anatomical changes of SN, detected via the SWI sequence at 7T, may distinguish MSA and PSP from CBD
[49][54], thereby confirming the pathological heterogeneity of these diseases. Of note, nigrosome-1 has also been visualized on 3D FLAIR images as an hyperintense structure within otherwise surrounding hypointense dorsolateral SN. Its loss can be used to predict presynaptic dopaminergic function and to diagnose PD with a high degree of accuracy
[50][55].
Recently, it has been reported that the combined visual analysis of SN (by using NM-MRI and nigrosome-1 imaging, displaying normal NM in SNc and nigrosome-1 loss) has enabled the distinction of MSA-P from PD and healthy controls
[51][56]. Moreover, it has also been described that a stratification of the swallow tail sign, using a scale on SWI-map imaging, may serve as a useful imaging biomarker regarding the differential diagnosis of Parkinsonism
[52][57]. However, the veracity of these results must be confirmed by larger cohort studies.
It is important to note that adequate visualization of the swallow tail sign requires targeted 3D high-resolution SWI. In the original publication, the parameters were: FEEPI, TR/TE 60/30, echo train length 5, flip angle 19°; 70 slices; voxel size 0.55 x 0.55 x 0.7 mm.[53]
4. Iron Imaging
Together with a degeneration of dopaminergic neurons, iron overload has been implicated in the pathology and pathogenesis of PD and PS. Iron deposition initially occurs in SN; however, abnormal iron levels have also been detected in the basal ganglia, thalamus, and cortex of PD patients
[54][58].
With the introduction of MRI, the in vivo characterization of brain iron content has become possible. The possibility of quantifying regional brain iron overload may provide more knowledge regarding the correlation between iron accumulation and parkinsonian symptoms. Indeed, extensive data have emphasized the importance of SN iron increase in PD patients compared to controls
[23][28][55][30,35,59]. From a technical perspective, the iron content can be assessed by evaluating T2 and T2* relaxation rates, using either magnitude (R2*) or phase (quantitative susceptibility mapping, QSM) imaging. Among these methods, R2 and R2* relaxometry (i.e.,: 1/T2*, proton transverse relaxation rate which reflects increased tissue iron content) considers heterogeneities from local and adjacent tissue as being more susceptible to influence from disturbances due to calcification, micro bleeds, and myelinated fibers
[11]. The R2* values in the SNc have been reported to be significantly higher in de novo PD patients with a gradual increase, which is related to disease progression
[56][57][60,61] (
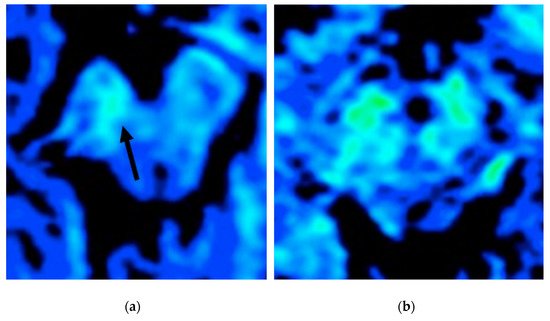
Figure 5).
Figure 5. T2* map study (color scale) of two patients with PD in an evaluation of iron deposition within the SN (blue: less iron deposition; green: more iron deposition). The patient in (a) has a more evident asymmetrical iron deposition when compared to the patient in (b).
Since correlations between motor symptoms and high levels of R2* values within the SNc have been reported in PD, and R2* changes rapidly with disease progression, these methods can also be used in the prospective evaluation of PD patients
[56][57][60,61]. Moreover, it has been reported that PD patients with early gait freezing pattern will have higher iron content, as evaluated by means of R2* relaxometry in the SNc, in comparison to those who do not
[58][62].
Furthermore, QSM provides a direct measure of the local heterogeneities of the magnetic field by using a deconvolution method, which assists in eliminating the susceptibilities of surrounding structures
[59][63]. It has been demonstrated that QSM is more sensitive than R2* in identifying iron overload in PD
[59][60][61][63,64,65], even in the prodromal stage of the disease
[62][66]. Values from QSM correlate with disease condition and duration
[60][61][63][64][64,65,67,68], and they distinguish PD and PS
[65][69]. Moreover, QSM can address iron variation within the SN
[66][70] and lateral asymmetry of iron deposition, which is related to a manifestation of asymmetric signs and symptoms in PD
[17][24]. When QSM is used in early- and advanced-stage PD patients, it is of note that it has been demonstrated that iron deposition affected SNc exclusively in the early stages of the disease, while in the late PD stage, iron deposition involved other regions, concomitant with SNc
[67][71]. This latter finding indicates that QSM is a tool with which to monitor iron deposition and disease progression in PD. Specifically, changes in iron seem to be limited to the ventral aspect of SN
[66][70], which has been reported to degenerate early in the course of the disease
[68][72]. According to the distribution of the pathological involvement distinguishing the various forms of Parkinsonism, red and subtalamic nuclei are involved in PSP, together with SN, while iron deposition in MSA is significantly higher in the putamen
[69][73]. Finally, all Parkinsonisms have been demonstrated to display increased susceptibility in the subcortical structures, thereby reflecting distinct topographical patterns of abnormal brain iron accumulation
[70][74].
Both QSM and R2* may be effective tools in the differential diagnoses of degenerative PS, a fact that permits the tracking of dynamic changes that are associated with the pathological progression of these disorders. In addition, while QSM is more sensitive to the iron content of SN, R2* can be said to reflect pathological features, such as α-synuclein, in addition to iron deposits
[71][75].