
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hidenori Suzuki | + 1245 word(s) | 1245 | 2021-07-13 11:24:46 | | | |
| 2 | Enzi Gong | Meta information modification | 1245 | 2021-07-14 05:45:37 | | |
Video Upload Options
Spinal cord injuries (SCI) are a devastating event and can lead to physical, psychosocial, and vocational implications for patients and their family. In the United States, approximately 288,000 individuals are estimated to suffer from symptoms caused by SCI, and a recent survey showed the annual incidence of SCI to be approximately 54 cases per one million people.
1. Overview
Chronic spinal cord injury (SCI) is a catastrophic condition associated with significant neurological deficit and social and financial burdens. It is currently being managed symptomatically with no real therapeutic strategies available. In recent years, a number of innovative regenerative strategies have emerged and have been continuously investigated in clinical trials. In addition, several more are coming down the translational pipeline. Among ongoing and completed trials are those reporting the use of mesenchymal stem cells, neural stem/progenitor cells, induced pluripotent stem cells, olfactory ensheathing cells, and Schwann cells. The advancements in stem cell technology, combined with the powerful neuroimaging modalities, can now accelerate the pathway of promising novel therapeutic strategies from bench to bedside. Various combinations of different molecular therapies have been combined with supportive scaffolds to facilitate favorable cell–material interactions. In this review, we summarized some of the most recent insights into the preclinical and clinical studies using stem cells and other supportive drugs to unlock the microenvironment in chronic SCI to treat patients with this condition. Successful future therapies will require these stem cells and other synergistic approaches to address the persistent barriers to regeneration, including glial scarring, loss of structural framework, and immunorejection.
2. Spinal Cord Injuries
3. Barriers to Regeneration and Pathophysiology of Chronic SCI
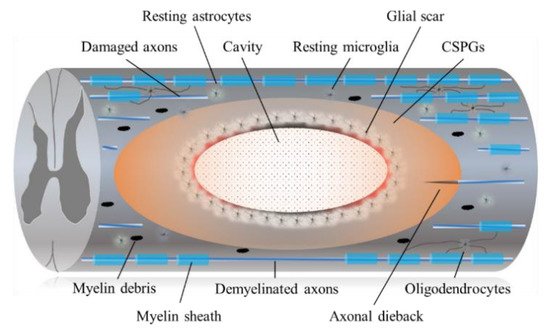
3.1. Astrocytic and Fibrotic Scar
3.2. CSPGs and Chondroitinase ABC (ChABC)
4. Conclusions
References
- Fehlings, M.G.; Martin, A.R.; Tetreault, L.A.; Aarabi, B.; Anderson, P.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Chiba, K.; Dettori, J.R.; et al. A clinical practice guideline for the management of patients with acute spinal cord injury: Recommendations on the role of baseline magnetic resonance imaging in clinical decision making and outcome prediction. Global Spine J. 2017, 7, 221s–230s.
- Fehlings, M.G.; Kim, K.D.; Aarabi, B.; Rizzo, M.; Lisa, M.B.; McKerracher, L.; Vaccaro, A.R.; David, O.; Okonkwo, D.O. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: Design of the SPinal Cord Injury Rho Inhibition InvestiGation (SPRING) clinical trial. J. Neurotrauma 2018, 35, 1049–1056.
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol 2014, 6, 309–331.
- Ahuja, C.; Fehlings, M.G. Concise review: Bridging the gap: Novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl. Med. 2016, 5, 914–924.
- Zweckberger, K.; Ahuja, C.S.; Liu, Y.; Wang, J.; Fehlings, M.G. Self-assembling peptides optimize the post-traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta. Biomater. 2016, 42, 77–89.
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016, 6, 1–8.
- Lu, P.; Kadoya, K.; Tuszynski, M.H. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr. Opin. Neurobiol. 2014, 27, 103–109.
- Spinal cord injury (SCI) 2016 facts and figures at a glance. J. Spinal Cord Med. 2016, 9, 493–494.
- Suzuki, H.; Ahuja, C.S.; Salewski, R.P.; Li, L.; Satkunendrarajah, K.; Nagoshi, N.; Shibata, S.; Fehlings, M.G. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS ONE 2017, 12, e0182339.
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389.
- Salewski, R.P.; Mitchell, R.A.; Shen, C.; Fehlings, M.G. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015, 24, 36–50.
- Wilcox, J.T.; Satkunendrarajah, K.; Zuccato, J.A.; Nassiri, F.; Fehlings, M.G. Neural precursor cell transplantation enhances functional recovery and reduces astrogliosis in bilateral compressive/contusive cervical spinal cord injury. Stem Cells Transl. Med. 2014, 3, 1148–1159.
- Ramon y Cajal, S. Degeneration and Regeneration of the Nervous System; Oxford University Press: London, UK, 1928.
- Cafferty, W.B.; Duffy, P.; Huebner, E.; Strittmatter, S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 2010, 30, 1825–1837.
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439.
- Forgione, N.; Fehlings, M.G. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014, 82, e535–e539.
- Moreau-Fauvarque, C.; Kumanogoh, A.; Camand, E.; Jaillard, C.; Barbin, G.; Boquet, I.; Love, C.; Jones, E.Y.; Kikutani, H.; Lubetzki, C.; et al. The transmembrane semaphoring Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 2003, 23, 9229–9239.
- Dubreuil, C.I.; Winton, M.J.; McKerracher, L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J. Cell Biol. 2003, 162, 233–243.
- Lord-Fontaine, S.; Yang, F.; Diep, Q.; Dergham, P.; Munzer, S.; Tremblay, P.; McKerracher, L. Local inhibition of Rho signaling by cell-permeable recombinant protein BA-210 prevents secondary damage and promotes functional recovery following acute spinal cord injury. J. Neurotrauma 2008, 25, 1309–1322.
- Fehlings, M.G.; Theodore, N.; Harrop, J.; Maurais, G.; Kuntz, C.; Shaffrey, C.I.; Kwon, B.K.; Chapman, J.; Yee, A.; Tighe, A.; et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J. Neurotrauma 2011, 28, 787–796.
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Myelin damage and repair in pathologic CNS: Challenges and prospects. Front. Mol. Neurosci. 2015, 8, 35.
- Avram, S.; Shaposhnikov, S.; Buiu, C.; Mernea, M. Chondroitin sulfate proteoglycans: Structure-function relationship with implication in neural development and brain disorders. Biomed. Res. Int. 2014, 2014, 642798.
- Bradbury, E.J.; Moon, L.D.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640.
- Jones, L.L.; Sajed, D.; Tuszynski, M. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: A balance of permissiveness and inhibition. J. Neurosci. 2003, 23, 9276–9288.
- Ikegami, T.; Nakamura, M.; Yamane, J.; Katoh, H.; Okada, S.; Iwanami, A.; Watanabe, K.; Ishii, K.; Kato, F.; Fujita, H.; et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur. J. Neurosci. 2005, 22, 3036–3046.
- Carter, L.M.; Stephen, B.; McMahon, S.B.; Bradbury, E.J. Delayed treatment with chondroitinase ABC reverses chronic atrophy of rubrospinal neurons following spinal cord injury. Exp. Neurol. 2011, 228, 149–156.
- Bartus, K.; James, N.D.; Didangelos, A.; Bosch, K.D.; Verhaagen, J.; Yáñez-Muñoz, R.J.; Rogers, J.H.; Schneider, B.J.; Muir, E.M.; Bradbury, E.J. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. 2014, 34, 4822–4836.




