Spinal cord injuries (SCI) are a devastating event and can lead to physical, psychosocial, and vocational implications for patients and their family. In the United States, approximately 288,000 individuals are estimated to suffer from symptoms caused by SCI, and a recent survey showed the annual incidence of SCI to be approximately 54 cases per one million people.
1. Overview
Chronic spinal cord injury (SCI) is a catastrophic condition associated with significant neurological deficit and social and financial burdens. It is currently being managed symptomatically with no real therapeutic strategies available. In recent years, a number of innovative regenerative strategies have emerged and have been continuously investigated in clinical trials. In addition, several more are coming down the translational pipeline. Among ongoing and completed trials are those reporting the use of mesenchymal stem cells, neural stem/progenitor cells, induced pluripotent stem cells, olfactory ensheathing cells, and Schwann cells. The advancements in stem cell technology, combined with the powerful neuroimaging modalities, can now accelerate the pathway of promising novel therapeutic strategies from bench to bedside. Various combinations of different molecular therapies have been combined with supportive scaffolds to facilitate favorable cell–material interactions. In this review, we summarized some of the most recent insights into the preclinical and clinical studies using stem cells and other supportive drugs to unlock the microenvironment in chronic SCI to treat patients with this condition. Successful future therapies will require these stem cells and other synergistic approaches to address the persistent barriers to regeneration, including glial scarring, loss of structural framework, and immunorejection.
2. Spinal Cord Injuries
Spinal cord injuries (SCI) are a devastating event and can lead to physical, psychosocial, and vocational implications for patients and their family. In the United States, approximately 288,000 individuals are estimated to suffer from symptoms caused by SCI, and a recent survey showed the annual incidence of SCI to be approximately 54 cases per one million people [
1,
2]. Worldwide, the estimated incidence of SCI ranges from 250,000–500,000 individuals per year [
3]. The majority of neuroregenerative therapy has focused on treating patients in the acute or subacute periods. In the acute to subacute phase, salvageable neuronal cells may still exist and the glial scar has not yet been established [
4,
5,
6,
7]. Unfortunately, 95% of patients with SCI are in the chronic phase of their injury [
8].
Despite this pressing need, one of the greatest challenges in developing an effective therapy for chronic SCI has been the inhibitory microenvironment of the injured spinal cord. After SCI, astrocytes activate, proliferate, and migrate to the perilesional region where they form a dense interwoven network of processes and deposit chondroitin sulfate proteoglycans (CSPGs) into the extracellular matrix. Dystrophic axons surround the injury epicenter and become trapped in the dense meshwork of scar tissue [
9].
Various cell populations can be used for the treatment of chronic SCI. Concurrently, several clinical trials using stem cells are underway around the world [ClinicalTrials.gov. Available online:
https://www.clinicaltrials.gov/ (accessed on 1 June 2021)]. Among them, exogenous neural stem cell (NSC) therapies are particularly promising as these cells have the potential to differentiate into all three neuroglial lineages (i.e., neurons, astrocytes, and oligodendrocytes) to regenerate neural circuits, remyelinate denuded axons, and provide trophic support to endogenous cells [
7,
9,
10,
11,
12].
3. Barriers to Regeneration and Pathophysiology of Chronic SCI
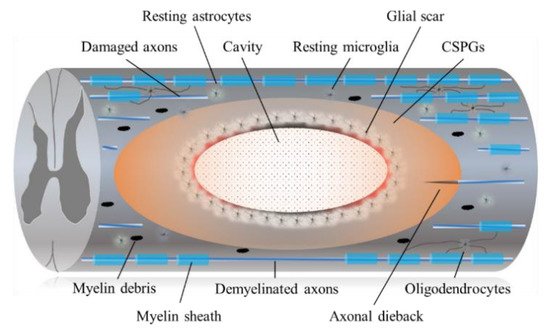
It is widely recognized that regeneration of the adult mammalian central nervous system (CNS), including the spinal cord, is difficult due to its limited plasticity [
13]. In the epicenter of a CNS lesion, a cavitation occurs that is surrounded by connective scar tissues containing cerebrospinal fluid. The phenotype of reactive astrocytes changes into scar-forming astrocytes that impede regenerating axons from crossing the lesion. Some inflammatory immune cells remain around the lesion even in the chronic phase of SCI (
Figure 1).
Figure 1. The diagram shows the pathophysiological events in the chronic SCI. Injury to the SCI results in death of neuronal as well as glial cells. Progressive demyelination results in degeneration of axonal fibers that leads to disruption of axo-glial signaling. A cavitation has occurred in the epicenter. Hypertrophic astrocytes with very long processes over the tips of non-regenerating fibers form a barrier known as a glial barrier or a glial wall around the cavitation. In response to damage/injury, microglial cells transform into active phagocytic microglia and exhibit chemotaxis (migrates and accumulates at the site of injury). The presence of CSPGs creates an inhibitory environment for axonal regeneration, which leads to failure of axonal growth cones at the injured site of CNS. In addition, CSPG also inhibits the migration and differentiation of oligodendrocyte progenitor cells.
3.1. Astrocytic and Fibrotic Scar
Astrocytes proliferate and tightly interweave their extended processes around the perilesional region in an attempt to wall off the injury epicenter. Astrocytes, pericytes, and ependymal cells generate dense deposits of CSPGs as part of the fibrous scar, which bind leukocyte common antigen-related receptors such as protein tyrosine phosphatases. This activates GTPase RhoA and its downstream effector, rho-associated protein kinase (ROCK), leading to the collapse of the axonal growth cone and regenerative failure [
14,
15,
16,
17]. C3 transferase, an enzyme derived from Clostridium botulinum, locks RhoA in the inactive state and thereby inhibits Rho signaling. C3 transferase has been shown to promote axonal outgrowth on inhibitory substrates, both in vitro and in vivo [
18,
19]. The SPinal Cord Injury Rho INhibition InvestiGation (SPRING) clinical trial is now underway (NCT02669849) for acute SCI [
2,
20].
3.2. CSPGs and Chondroitinase ABC (ChABC)
CSPGs are a class of extracellular matrix molecule proteoglycans that are widely expressed within the CNS and that can be synthesized by all neural cell types [
21]. CSPGs are highly upregulated in the glial scar after injury to the nervous system. In addition, they are mostly inhibitory and have been shown to hinder regeneration of axons across lesions in chronic SCI [
22]. ChABC is a bacterial enzyme shown to effectively degrade CSPGs, including NG2, and to promote functional gains in mouse models after intrathecal administration [
23,
24]. Evidence also shows that co-administration of ChABC with neural precursor cells enhances transplant survival and remyelination of host axons [
25,
26]. More recently, large-scale CSPG digestion by direct lentiviral ChABC gene delivery into rat spinal cords resulted in a reduced cavitation volume and an enhanced preservation of axons. Treated rats also displayed an improved sensorimotor function on behavioral and electrophysiological assessments [
27]. We also reported that ChABC administration reduced chronic injury scar and resulted in significantly improved NSCs derived from induced pluripotent stem cell (iPSC-NSC) survival with clear differentiation into all three neuroglial lineages. The chronically injured spinal cord can be ‘unlocked’ by ChABC pretreatment to produce a microenvironment conducive to regenerative iPSC therapy [
9]. ChABC is an exciting therapy for which the optimal delivery modality remains to be elucidated. Future avenues of chronic SCI research may include exploration of human CNS-specific analogs of ChABC and its development.
4. Conclusions
Currently, numerous clinical and experimental studies have shown positive results in terms of functional improvement with stem cell treatment in chronic SCI. Various designs of chronic SCI trials need to be performed. However, human chronic SCI trials are not easily enforced because of some inherent limitations. First, comparison between treatment and control groups is difficult because of ethical aspects, and, in terms of safety and efficacy, the results of animal experiments cannot be directly applied to humans. Nevertheless, a lot of basic research and clinical trials of stem cell therapy have already been performed, and promising results have also been reported. We are convinced that stem cell therapy will provide the drastic treatment needed for chronic SCI patients in the near future.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22147435