
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Melanie S. Matos | + 9443 word(s) | 9443 | 2021-07-07 04:45:03 | | | |
| 2 | Bruce Ren | -21 word(s) | 9422 | 2021-07-14 03:35:58 | | |
Video Upload Options
Inflammation is a crucial and complex process that reestablishes the physiological state after a noxious stimulus. In pathological conditions the inflammatory state may persist, leading to chronic inflammation and causing tissue damage. Sesquiterpene lactones (SLs) are composed of a large and diverse group of highly bioactive plant secondary metabolites, characterized by a 15-carbon backbone structure. In recent years, the interest in SLs has risen due to their vast array of biological activities beneficial for human health. The anti-inflammatory potential of these compounds results from their ability to target and inhibit various key pro-inflammatory molecules enrolled in diverse inflammatory pathways, and prevent or reduce the inflammatory damage on tissues. Research on the anti-inflammatory mechanisms of SLs has thrived over the last years, and numerous compounds from diverse plants have been studied, using in silico, in vitro, and in vivo assays. Besides their anti-inflammatory potential, their cytotoxicity, structure–activity relationships, and pharmacokinetics have been investigated.
1. Sesquiterpene Lactones
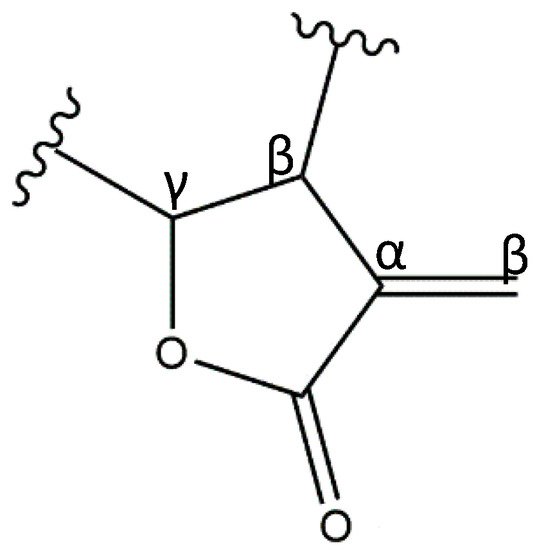
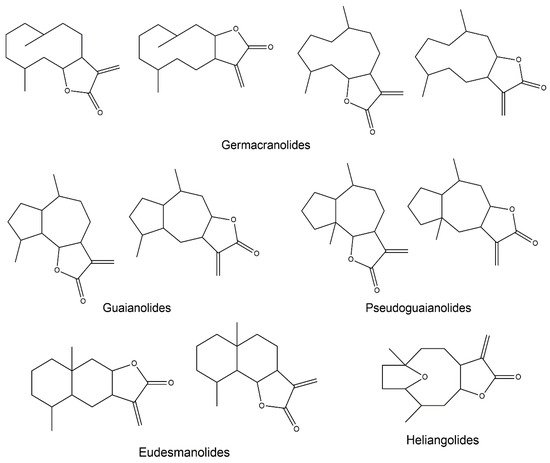
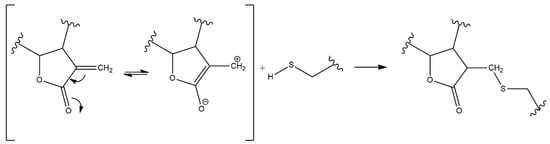
2. Anti-Inflammatory Potential of Sesquiterpene Lactones
2.1. General Notes on Inflammation
2.2. SL-Containing Extracts
| Source | Main SLs | Model | Extract Concentration Range | Inflammatory Pathways | Consequences | References | |
|---|---|---|---|---|---|---|---|
| Cichorium intybus L. | Dihydrolactucin, lactucin, deoxylactucin, jacquinelin and dihydrolactucopicrin | In vitro | RAW 264.7 murine macrophages + LPS | IC50 (μg/mL): 117 for COX-2; 39 for iNOS; 48 for TNF-α; 22 for IL-1β; 21 for NO | - | ↓ COX-2, iNOS, TNF-α, IL-1β, NO | [17] |
| In vivo | Paw edema model: Wistar rats + carrageenan (subcutaneous) | 50–100 mg/kg (oral administration) | - | ↓ paw volume (edema) | |||
| Arthritis model: Wistar rats + collagen (intravenous) | 200 mg/kg (oral administration) | - | |||||
| Artemisia leucodes L. | Leukomisin and austricin | In vitro | RAW264.7 murine macrophages + LPS | 2–100 μg/mL | - | ↓ COX-2, iNOS, IL-1β, NO | [18] |
| COX-1 and -2 enzymatic assay | 45–225 μg/mL | ↓ COX-2 | |||||
| In vivo | Paw edema model: Wistar rats + carrageenan (subcutaneous) | 50–200 mg/kg (oral administration) | ↓ paw edema | ||||
| Chronic inflammation model: Wistar rats + cotton implant granuloma test | 50 mg/kg (oral administration) | ↓ granuloma and inflammatory cell infiltrate | |||||
| Artemisia khorassanica L. | Unspecified | In vitro | J774A.1 murine macrophages + LPS | 10–100 μg/mL | ↓ NF-κB | ↓ COX-2, PGE2, iNOS, NO, TNF-α and IL-1β | [19] |
| Artemisia sps (A. kopetdaghensis, A. santolina, A. Sieberi, A. Fragrans, A. Absinthium, A. ciniformis) |
Saturated, unsaturated and unusual SLs | In vitro | J774A.1 murine macrophages + LPS | 10–100 μg/mL | - | ↓ COX-2, PGE2, iNOS and NO | [20] |
| Eupatorium perfoliatum L. | Diguaiaperfolin (dimeric guaianolide) and Eupafolin (flavonoid) | In vitro | RAW264.7 murine macrophages + LPS | 1–100 μg/mL | - | ↓ NO, CSF-3, IL-6, IL-1α, IL-1β, TNF, Chemokine (C-C motif) ligand (CCL)-2, CCL22 and CXCL10 | [21] |
| Xanthium spinosum L. | Ziniolide | In vitro | Rat polymorphonuclear leukocytes (PMNLs) + ionophore A23187 and Ca2+ | 0–100 μg/mL | ↓ NF-κB and arachidonic acid | ↓ 5-LOX | [22] |
| Human platelets + ionophore A23187 | 25–200 μg/mL | ↓ COX-1 and 12-LOX; ↑ 15(S)-HETE | |||||
| HeLa cells + Phorbol 12-myristate 13-acetate (PMA) | 12.5–100 μg/mL | ↓ NF-κB activation | |||||
| Arnica montana L. | Helenalin and dihydrohelenalin ester derivatives | In vitro | Jurkat T cells + TNF-α or PMA | 0.5–10 μL/mL | ↓ NF-κB and NFAT | ↓ NF-κB and NFAT DNA-binding | [23] |
| Human PBMCs from healthy donors + LPS | 0.001–10 μL/mL | ↓ TNF-α and IL-1β | |||||
| Centaurea L. species (C. aphrodisea, C. athoa, C. hyalolepis, C. iberica, C. polyclada) | SL fraction (athoin, 14-O-acetylathoin and methyl-14-O-acetylathoin-12-oate in C. athoa) | In vitro | SW1353 human chondrosarcoma cells + PMA | 0–100 μg/mL | ↓ NF-κB | ↓ NF-κB activity | [24][25] |
| RAW264.7 murine macrophages + LPS | ↓ NO | ||||||
| In vivo | Paw edema model: Wistar rats + carrageenan (subcutaneous) | 6.75–50 mg/kg (oral administration) | ↓ edema | ||||
| Inula helenium L | Alantolactone and isoalantolactone | In vitro | bEnd.3 mouse endothelial cells + TNF-α | 0.6–2.4 μg/mL | ↓ NF-κB and MAPKs | ↓NF-κB inhibitor (IκB)-α, NF-κB p65, p38 and c-Jun N-terminal kinase (JNK) phosphorylation | [26] |
| RAW264.7 murine macrophages + LPS | ↓ IL-1, IL-6 and iNOS | ||||||
| Primary synovial fibroblasts from rheumatoid arthritis patients + TNF-α | ↓ IL-1, MCP-1 and MMP-3 | ||||||
| In vivo | Adjuvant-induced mice arthritis model | 12.5–50 mg/kg (oral administration) | ↓ paw swelling | ||||
| Collagen-induced mice arthritis model | |||||||
| Arctium lappa L. | Onopordopicrin | In vivo | Colitis model: Wistar rats + Trinitrobenzene Sulfonic Acid (TNBS) (enteral instillation) | 25–50 mg/kg (oral administration) | - | ↓ TNF-α and COX-2; ↓ histological damage; ↓ mucin layer loss; ↓ neutrophil infiltration | [27] |
| Vernonia scorpioides L. | Diacethylpiptocarphol and related hirsutinolides |
In vivo | Acute ear edema model: Swiss mice + 12-O-tetradecanoylphorbol acetate (TPA) (topical) | 0.003–1 mg (topical) | ↓ NF-κB | ↓ neutrophil infiltration, edema and epidermal proliferation | [28] |
| Chronic ear edema model: Swiss mice + arachidonic acid (topical) or croton oil (topical) | 1 mg (topical) | ||||||
2.3. Germacranolides
2.3.1. Parthenolide
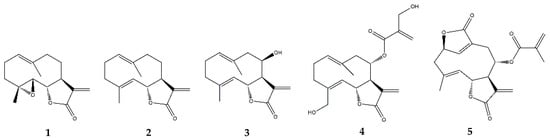
2.3.2. Costunolide
2.3.3. Other Germacranolides
2.4. Guaianolides
2.5. Eudesmanolides
2.6. Heliangolides
2.7. Pseudoguaianolides
2.8. Other SL Subclasses
| SL Subclass |
Compound Name (ID Number) |
Model | Compound Concentration Ranges Tested | Inflammatory Pathways | Consequences | References | |
|---|---|---|---|---|---|---|---|
| Germacranolides | Parthenolide (1) |
In vitro | Rat aortic smooth muscle cells + LPS/IFN-γ | 3–30 μM | ↓ NF-κB | ↓ iNOS and NO release | [31] |
| In vitro | BV2 mouse microglia + LPS | 5 μM | ↓ NF-κB | ↓ IL-6 and TNF-α | [32] | ||
| In vitro | Rat primary neural-glial cells + LPS | 403 μM | ↓ NF-κB, NF-IL6, Nrf-1 and PGC1α | ↓ IL-6 and TNF-α | [33] | ||
| In vivo | Wistar rats + LPS (intraperitoneal injection) | 1 mg/kg (intraperitoneal injection) | ↓ IL-6 and TNF-α in plasma; ↓ COX-2, NF-IL6, SOCS3, IκBα and Tribbles pseudokinase 1 (Trib1) in hypothalamus; ↓ fever | ||||
| In vitro | Jurkat T cells and primary peripheral human T cells + PMA/ionomycin or anti-CD3/CD28 | 1.25–5 μM | ↓ NF-κB and AP-1 | ↓ IL-4, IL-2 and IFN-γ | [34] | ||
| Primary peripheral human T cells + PMA/ionomycin or anti-CD3/CD28 | |||||||
| In vitro | Blood from healthy donors + PMA/ionomycin | 10–500 μM | - | ↓ IL-2; ↓ T-lymphocyte activation | [35] | ||
| In vitro | Human THP-1 monocytes + LPS | 0.75–12 μM | ↓ NF-κB and MAPKs | ↓ IL-6, TNF-α, IL-1β, IL-8, IL-18 and NO; ↓ iNOS, TLR4 and TRAF6 | [36] | ||
| Human primary monocytes + LPS | |||||||
| In vivo | Hindpaw edema model: Holtzman rats + carrageenan (subcutaneous injection) | 5–20 mg/kg (intraperitoneal injection) | - | ↓ Hyperalgesia and edema | [37] | ||
| In vitro | HepG2 human hepatocytes + IL-6, oncostatin M or leukemia inhibitory factor | 5 μM | ↓ STAT3 and JAKs | ↓ STAT3 phosphorylation, dimerization and activity | [39] | ||
| Costunolide (2) |
In vitro | Human THP-1 monocytes + IL-6 | 6–25 μM | ↓ IL-6/STAT3 and JAKs | ↓ MCP-1, CXCL10, ICAM-1; ↓ STAT3 phosphorylation and DNA-binding activity; ↓ Intracellular GSH | [40] | |
| In vitro | RAW264.7 murine macrophages + LPS | 0.1–1 μM | ↑ Nrf-2; ↓ NF-κB | ↑ HO-1; ↓ IL-6 and TNF-α | [38] | ||
| In vitro | Human keratinocytes from healthy donors + IL-22, IFN-γ or TNF-α | 12.5 μM | ↓ STAT3 and STAT1 | ↓ Intracellular GSH; ↓ CCL2, CXCL10, ICAM-1 and SOCS3; ↑ Epidermal growth factor receptor (EGFR) and Erk1/2 | [41] | ||
| In vitro | RAW264.7 murine macrophages + LPS | 0.1–3 μM | ↓ AP-1 and MAPKs | ↓ IL-1β | [42] | ||
| In vitro | Primary rat chondrocytes + IL-1β | 2–6 μM | ↓ NF-κB and Wnt/β-catenin; ↑ SOX-9 | ↓ MMP-3, MMP-9, MMP-13, iNOS, COX-2 and IL-6; ↑ collagen II | [43] | ||
| In vivo | Sprague-Dawley rats (surgically induced osteoarthritis model) | 6 μM (intra-articular injection) | attenuation of cartilage degeneration | ||||
| In vivo | Angiogenesis model: Swiss albino mice + polyester-polyurethane sponge implants | 5–20 mg/kg (cannula) | - | ↓ Angiogenesis, macrophage and neutrophil accumulation, and collagen deposition; ↓ IL-1β, IL-6, IL-17, TNF-α, TGF-β; ↑ IL-10 | [44] | ||
| Eupatolide (3) | In vitro | RAW264.7 murine macrophages + LPS | 0.1–10 μM | ↓ NF-κB, AP-1, MAPKs, Akt | ↓ COX-2, PGE2, iNOS, NO and TRAF6 | [45] | |
| Human embryonic kidney (HEK)-293 cells + LPS | ↑ proteossomal degradation of TRAF6 | ||||||
| Onopordopicrin (4) | In vitro | NIH-3T3 cell line + TNF-α | IC50 (μM): 8.6 for NF-κB; 15.3 for STAT3; EC50 (μM): 2.2 for Nrf-2 | ↓ NF-κB and STAT3; ↑ Nrf-2 | ↓ NF-κB activity | [46] | |
| HeLa cell line + IFN-γ | ↓ STAT3 activity | ||||||
| HaCaT keratinocytes | ↑ Nrf-2 activity | ||||||
| Deoxyelephantopin (5) | In vitro | RAW264.7 murine macrophages + LPS | 2.5–10 μM | - | ↓ high mobility group box (HMGB) 1, pyruvate kinase M2 (PKM2), glucose transporter 1 (GLUT1), lactate dehydrogenase A (LDHA) and phosphoinositide-dependent kinase 1 (PDK1) and IL-1β | [47] | |
| In vivo | C57BL/6J mice + LPS (intraperitoneal injection) | 10 mg/kg (intraperitoneal injection) | - | ↓ endotoxic shock and sepsis | |||
| Guaianolides | Dehydrocostuslactone (6) | In vitro | THP-1 human cells + IL-6 | 6–25 μM | ↓ IL-6/STAT3 and JAKs | ↓ MCP-1, CXCL10, ICAM-1; ↓ STAT3 phosphorylation and DNA-binding activity; ↓ Intracellular GSH | [40] |
| In vitro | Human keratinocytes from healthy donors + IL-22, IFN-γ or TNF-α | 12.5 μM | ↓ STAT3 and STAT1 | ↓ Intracellular GSH; ↓ CCL2, CXCL10, ICAM-1 and SOCS3; ↑ EGFR and Erk1/2 | [41] | ||
| In vivo | Colitis model: BALB/c mice + Dextran sulfate sodium (DSS) (oral administration) | 10–20 mg/kg | ↓ IL-6/STAT3 | ↓ TNF-α, IL-1β, MPO, SOD, IL-6, IL-17, IL-23, COX-2, iNOS | [50] | ||
| In vitro | RAW 264.7 macrophages + LPS | 10–20 μM | ↓ MyD88/TRIF; ↓ NF-κB; ↓ IRF-3 | ↓ COX-2, INF-β, IP-10 | [51] | ||
| Micheliolide (7) | In vitro | BV2 microglia cells + LPS | 1–10 μM | ↓ NF-κB; ↓ PI3K/Akt ↓ MAPKs |
↓ TNF-α, IL-6, IL-1β, COX-2, iNOS | [52] | |
| In vitro | RAW 264.7 macrophages + LPS | 1–10 μM | ↓ NF-κB | ↓ IL-6, TNF-α, IL-1β | [53] | ||
| In vitro | RAW264.7 macrophages + LPS | 0–10 μM | ↓ NF-κB; ↓ PI3K/Akt | ↓ IL-6, TNF-α, MCP-1, INF-β and IL-1β | [54] | ||
| Human dendritic cells and monocytes + LPS | ↓ IL-6, TNF-α, MCP-1, INF-β | ||||||
| In vivo | Arthritis model: DBA/1 mice + collagen (intradermal injection) | 30 mg/kg (intraperitoneal injection) | - | ↓ TIMP-1, M-CSF, ICAM-1, INF-γ | [55] | ||
| Cynaropicrin (8) | In vitro | RAW 264.7 macrophages + LPS | 0–35 μM | - | ↓ TNF-α and NO | [56] | |
| Human macrophages U937 + LPS | |||||||
| Primary splenocytes from mice + concanavalin A, phytohemagglutinin and LPS | ↓ lymphocyte proliferation | ||||||
| Arglabin (9) | In vitro | Peritoneal macrophages from ApoE2.Ki mice + LPS and cholesterol crystals | 50 nM | ↓ NF-κB; ↓ NLRP3 |
↓ IL-1α, IL-1β, IL-18 | [57] | |
| 11β,13-dihydrolactucin (10) | In vitro | Yeast S. cerevisiae + MnCl2 | 0.36–18 μM | ↓ Calcineurin-Crz1 (NFAT) | ↓ NFAT nuclear translocation and transcriptional activity | [58] | |
| 8-deoxylactucin (11) | In vitro | Human colon-cancer cells HT29 + TNF-α | 115 μM | ↓ NF-κB | ↓ PGE2 | [59] | |
| Eudesmanolides | Alantolactone (12) | In vitro | bEnd.3 mouse endothelial cells + TNF-α | 2.6–10.3 μM | ↓ NF-κB and MAPKs | ↓ IκBα, NF-κB p65, p38 and JNK phosphorylation | [26] |
| RAW264.7 murine macrophages + LPS; | ↓ IL-1, IL-6 and iNOS | ||||||
| Primary synovial fibroblasts from rheumatoid arthritis patients + TNF-α | ↓ IL-1, MCP-1 and MMP-3 | ||||||
| In vitro | RAW 264.7 macrophages + LPS | 1.25–10 μM | ↓ NF-κB; ↓ MyD88 |
↓ iNOS, COX-2, TNF-α | [60] | ||
| In vivo | Colitis model: C57BL/6 mice + DSS (oral administration) | 50 mg/kg (oral administration) | ↓ NF-κB; ↑ hPXR |
↓ iNOS, ICAM-1, COX-2, TNF-α, IFN-γ, IL-6 | [61] | ||
| Isoalantolactone (13) | In vitro | bEnd.3 mouse endothelial cells + TNF-α | 2.6–10.3 μM | ↓ NF-κB and MAPKs | ↓ IκBα, NF-κB p65, p38 and JNK phosphorylation | [26] | |
| RAW264.7 murine macrophages + LPS | ↓ IL-1, IL-6 and iNOS | ||||||
| Primary synovial fibroblasts from rheumatoid arthritis patients + TNF-α | ↓ IL-1, MCP-1 and MMP-3 | ||||||
| In vitro | 293T cells + TNF-α | 2.5–10 μM | ↓ NF-κB and MAPKs | ↓ UbcH5 | [62] | ||
| In vivo | Hepatitis model: BALB/c mice + TNF-α and D-galactosamine (D-GalN) (intraperitoneal injection) | 10 mg/kg (intraperitoneal injection) | ↓ serum alanine aminotransferase (ALT); ↓ hepatocyte damage; ↓ IL-6, MCP-1, ICAM-1 and VCAM-1 | ||||
| JEUD-38 (14) | In vitro | RAW 264.7 macrophages + LPS | 2.5–10 μM | ↓ NF-κB and MAPKs | ↓ iNOS | [63] | |
| 7-hydroxyfrullanolide (16) | In vitro | THP-1 cell + LPS | 0.3–100 μM | ↓ NF-κB | ↓ NF-κB activation and nuclear translocation | [64] | |
| HUVECs + LPS | ↓ ICAM-1, VCAM-1, E-selectin ↓ Monocyte adhesion |
||||||
| PBMCs + LPS | ↓ NF-κB-related gene expression | ||||||
| In vitro | PBMCs + LPS | 0.3–100 μM | - | ↓ IL-6 and TNF-α | [65] | ||
| Primary human synovial tissue cells | |||||||
| In vivo | Colitis model: BALB/c mice + DSS (oral administration) | 75 mg/kg (oral administration) | - | ↓ TNF-α and IL-6; ↓ Colonic edema; ↓ Shortening of the colon; ↓ hemoglobin and rectal bleeding; ↓ neutrophil infiltration |
|||
| Paw edema model: Wistar rats + carrageenan (subcutaneous injection) | 100 mg/kg | - | ↓ paw edema | ||||
| Arthritis model: DBA/1J mice + collagen (intradermal injection) | 25–75 mg/kg (oral administration) | - | ↓ joint deformities and bone destruction | ||||
| Santamarin (17) | In vitro | RAW264.7 macrophages + LPS | 5–40 μM | ↓ NF-κB; ↑ Nfr2 |
↓ COX-2 and iNOS; ↓ TNF-α, IL-1β ↑ HO-1 |
[66] | |
| Murine peritoneal macrophages + LPS | ↓ COX-2 and iNOS; ↓ TNF-α, IL-1β | ||||||
| Heliangolides | Lychnopholide (18) | In vitro | J774A.1 macrophages + INF-γ and LPS | 0.0125–0.2 μM | - | ↑ IL-10; ↓ NO |
[67] |
| Eremantholide (19) | J774A.1 macrophages + INF-γ and LPS | 0.625–10 μM | - | ↑ IL-10; ↓ TNF-α |
|||
| Budlein A (20) | In vitro | RAW264.7 + TNF-α or IL-1β | 2.7 × 104–26.7 μM | ↓ NF-κB | ↓ NF-κB activity | [68] | |
| In vivo | Arthritis model: C57BL/6 mice + methylated bovine serum albumin (intra-articular injection) | 1–10 mg/kg (oral administration) | ↓ edema; ↓ neutrophil and leukocyte infiltration; ↓ proteoglycan degradation; ↓ IL-33, TNF-α, IL-1β, COX-2 | ||||
| In vivo | Paw edema model: Swiss mice + carrageenan (subcutaneous injection) | 1–10 mg/kg | - | ↓ TNF-α, IL-1β; ↓ edema, and neutrophil infiltration; ↓ mechanical hypernocecipetion | [69] | ||
| In vivo | Gout arthritis model: Swiss mice + monosodium urate crystals (intra-articular injection) | 1–10 mg/kg (oral administration) | ↓ NF-κB; ↓ NLRP3 inflammasome |
↓ TNF-α and IL-1β; ↓ neutrophil recruitment; ↓ edema and mechanical hypersensitivity |
[70] | ||
| In vitro | Bone marrow derived macrophages (BMDMs) + LPS and monosodium urate crystals | 2.7–26.7 mM | ↓ TNF-α and IL-1β | ||||
| Diacethylpiptocarphol (21) | In vivo | Colitis model: BALB/c mice + DSS (oral administration) | 5 mg/kg (oral administration) | - | ↓ TNF-α; ↑ TGF-β; ↓ immune cell infiltration and tissue damage |
[71] | |
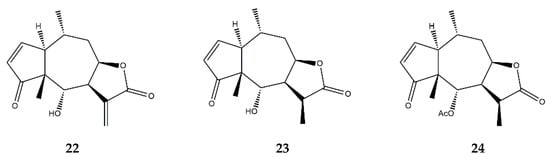
| Pseudoguaianolides | Helenalin (22) | In vitro | Jurkat T cells + TNF-α | 5–200 μM | ↓ NF-κB | ↓ NF-κB DNA-binding | [72] |
| In vitro | Jurkat T cells + TNF-α | 10 μM | ↓ NF-κB | ↓ NF-κB DNA-binding and nuclear translocation | [73] | ||
| In vitro | Jurkat CD4+ T-cells | 0.5–5 μM | ↓ NFAT ↓ NF-κB |
↓ IL-2 ↓ proliferation of CD4+ cells |
[23][74] | ||
| In vitro | THP-1 cells + LPS | 0.52–1.08 μM | ↓ NF-κB | ↓ IL-1α, IL-19, MCP-3, GM-CSF | [75] | ||
| In vitro | A2780 human ovarian cancer cell line | 0.5–2 μM | ↓ NF-κB | ↓ NF-κB p65 expression | [76] | ||
| 11α,13-dihydrohelenalin (23) | In vitro | PBMCs + LPS | 2–20 μM | ↓ NF-κB and NFAT | ↓ IL-2, IL-6, GM-CSF, TNF-α, INF-γ, iNOS | [77] | |
| Jurkat T-cells + LPS | ↓ NF-κB and NFAT levels | ||||||
| 11α,13-dihydrohelenalin–acetate (24) | In vitro | PBMCs + LPS | 2–20 μM | ↓ NF-κB and NFAT | ↓ IL-2, IL-6, GM-CSF, TNF-α, INF-γ, iNOS | ||
| Jurkat T-cells + LPS | ↓ NF-κB and NFAT levels | ||||||
| In vitro | Human granulocytes + Ionophore A23187 | 1–600 μM | ↓ Arachidonic Acid | ↓ Leukotriene C4 synthase; ↓ 5-lipooxygenase |
[78] | ||
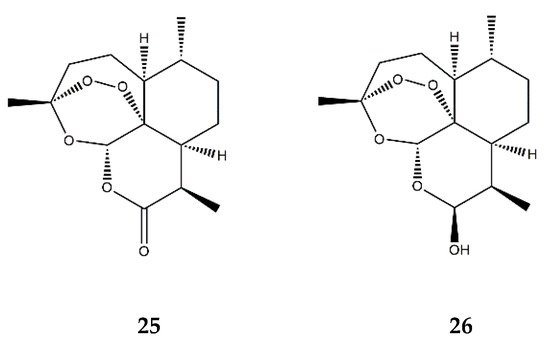
| Endoperoxide SL | Artemisinin (25) | In vitro | HUVECs + TNF-α | 50–200 μM | ↓ NF-κB; ↓ MAPKs | ↓ ICAM-1, VCAM-1; ↓ adhesion of monocytes | [80] |
| Dihydroartemisinin (26) | In vitro | RAW 264.7 macrophages + PMA | 5–25 μM | ↓ NF-κB, AP-1 and MAPKs | ↓ COX-2 | [81] | |
References
- Chaturvedi, D.; Dwivedi, P. Recent Developments on the Antidiabetic Sesquiterpene Lactones and Their Semisynthetic Analogues. In Discovery and Development of Antidiabetic Agents from Natural Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–205.
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700.
- Lopes, A.A.; Pina, E.S.; Silva, D.B.; Pereira, A.M.S.; Silva, M.F.D.G.F.D.; Da Costa, F.B.; Lopes, N.P.; Pupo, M.T. A biosynthetic pathway of sesquiterpene lactones in Smallanthus sonchifolius and their localization in leaf tissues by MALDI imaging. Chem. Commun. 2013, 49, 9989–9991.
- Ramirez, A.M.; Saillard, N.; Yang, T.; Franssen, M.C.R.; Bouwmeester, H.J.; Jongsma, M.A. Biosynthesis of Sesquiterpene Lactones in Pyrethrum (Tanacetum cinerariifolium). PLoS ONE 2013, 8, e65030.
- Majdi, M.; Liu, Q.; Karimzadeh, G.; Malboobi, M.A.; Beekwilder, J.; Cankar, K.; De Vos, R.; Todorović, S.; Simonović, A.; Bouwmeester, H. Biosynthesis and localization of parthenolide in glandular trichomes of feverfew (Tanacetum parthenium L. Schulz Bip.). Phytochemistry 2011, 72, 1739–1750.
- Amrehn, E.; Aschenbrenner, A.-K.; Heller, A.; Spring, O. Localization of sesquiterpene lactone biosynthesis in cells of capitate glandular trichomes of Helianthus annuus (Asteraceae). Protoplasma 2016, 253, 447–455.
- Hussien, T.; Mohamed, T.; Elshamy, A.; Moustafa, M.; El-Seedi, H.; Pare, P.; Hegazy, M.-E. Guaianolide Sesquiterpene Lactones from Centaurothamnus maximus. Molecules 2021, 26, 2055.
- Merfort, I. Perspectives on Sesquiterpene Lactones in Inflammation and Cancer. Curr. Drug Targets 2011, 12, 1560–1573.
- Hohmann, M.S.; Longhi-Balbinot, D.T.; Guazelli, C.F.; Navarro, S.A.; Zarpelon, A.C.; Casagrande, R.; Arakawa, N.S.; Verri, W.A. Sesquiterpene Lactones: Structural Diversity and Perspectives as Anti-Inflammatory Molecules. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 243–260.
- Schmidt, T.J. Structure-activity relationships of sesquiterpene lactones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 309–392.
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258.
- Padilla-Gonzalez, G.F.; Dos Santos, F.A.; Da Costa, F.B. Sesquiterpene Lactones: More Than Protective Plant Compounds with High Toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37.
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435.
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776.
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820.
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the asteraceae. Bot. Rev. 1982, 48, 121–594.
- Ripoll, C.; Schmidt, B.M.; Ilic, N.; Poulev, A.; Dey, M.; Kurmukov, A.G.; Raskin, I. Anti-inflammatory Effects of a Sesquiterpene Lactone Extract from Chicory (Cichorium intybus L.) Roots. Nat. Prod. Commun. 2007, 2, 717–722.
- Schmidt, B.; Belolipov, I.V.; Kurmukov, A.G.; Zakirov, S.; Raskin, L. Sesquiterpene Lactone Extract from Artemisia leucodes for Reducing Inflammation and Down-Regulating Pro-Inflammatory Gene Expression. US Patent US20080145465A1, 19 June 2008.
- Emami, S.A.; Rabe, S.Z.T.; Iranshahi, M.; Ahi, A.; Mahmoudi, M. Sesquiterpene lactone fraction fromArtemisia khorassanicainhibits inducible nitric oxide synthase and cyclooxygenase-2 expression through the inactivation of NF-κB. Immunopharmacol. Immunotoxicol. 2010, 32, 688–695.
- Zamani, S.; Emami, S.A.; Iranshahi, M.; Rabe, S.Z.T.; Mahmoudi, M. Sesquiterpene fractions of Artemisia plants as potent inhibitors of inducible nitric oxide synthase and cyclooxygenase-2 expression. Iran. J. Basic Med. Sci. 2019, 22, 774–780.
- Maas, M.; Deters, A.M.; Hensel, A. Anti-inflammatory activity of Eupatorium perfoliatum L. extracts, eupafolin, and dimeric guaianolide via iNOS inhibitory activity and modulation of inflammation-related cytokines and chemokines. J. Ethnopharmacol. 2011, 137, 371–381.
- Bader, A.; Giner, R.M.; Martini, F.; Schinella, G.R.; Ríos, J.L.; Braca, A.; Prieto, J.M. Modulation of COX, LOX and NFκB activities by Xanthium spinosum L. root extract and ziniolide. Fitoterapia 2013, 91, 284–289.
- Klaas, C.A.; Wagner, G.; Laufer, S.; Sosa, S.; Della Loggia, R.; Bomme, U.; Pahl, H.L.; Merfort, I. Studies on the Anti-Inflammatory Activity of Phytopharmaceuticals Prepared from Arnica Flowers. Planta Med. 2002, 68, 385–391.
- Erel, S.B.; Demir, S.; Nalbantsoy, A.; Ballar, P.; Khan, S.; Yavasoglu, N.U.K.; Karaalp, C. Bioactivity screening of fiveCentaureaspecies andin vivoanti-inflammatory activity ofC. athoa. Pharm. Biol. 2014, 52, 775–781.
- Demir, S.; Karaalp, C.; Bedir, E. Unusual sesquiterpenes from Centaurea athoa DC. Phytochem. Lett. 2016, 15, 245–250.
- Gao, S.; Wang, Q.; Tian, X.-H.; Li, H.-L.; Shen, Y.-H.; Xu, X.-K.; Wu, G.-Z.; Hu, Z.-L.; Zhang, W.-D. Total sesquiterpene lactones prepared from Inula helenium L. has potentials in prevention and therapy of rheumatoid arthritis. J. Ethnopharmacol. 2017, 196, 39–46.
- De Almeida, A.B.A.; Hidalgo, M.S.; Martín, A.R.; Luiz-Ferreira, A.; Trigo, J.R.; Vilegas, W.; Dos Santos, L.C.; Souza-Brito, A.R.M.; De La Lastra, C.A. Anti-inflammatory intestinal activity of Arctium lappa L. (Asteraceae) in TNBS colitis model. J. Ethnopharmacol. 2013, 146, 300–310.
- Rauh, L.K.; Horinouchi, C.D.; Loddi, A.M.; Pietrovski, E.F.; Neris, R.; Souza-Fonseca-Guimaraes, F.; Buchi, D.F.; Biavatti, M.W.; Otuki, M.F.; Cabrini, D.A. Effectiveness of Vernonia scorpioides ethanolic extract against skin inflammatory processes. J. Ethnopharmacol. 2011, 138, 390–397.
- Weng, H.; He, L.; Zheng, J.; Li, Q.; Liu, X.; Wang, D. Low Oral Bioavailability and Partial Gut Microbiotic and Phase II Metabolism of Brussels/Witloof Chicory Sesquiterpene Lactones in Healthy Humans. Nutrients 2020, 12, 3675.
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565.
- Wong, H.; Menendez, I.Y. Sesquiterpene Lactones Inhibit Inducible Nitric Oxide Synthase Gene Expression in Cultured Rat Aortic Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 1999, 262, 375–380.
- Magni, P.; Ruscica, M.; Dozio, E.; Rizzi, E.; Beretta, G.; Facino, R.M. Parthenolide Inhibits the LPS-induced Secretion of IL-6 and TNF-α and NF-κB Nuclear Translocation in BV-2 Microglia. Phytother. Res. 2012, 26, 1405–1409.
- Rummel, C.; Gerstberger, R.; Roth, J.; Hübschle, T. Parthenolide attenuates LPS-induced fever, circulating cytokines and markers of brain inflammation in rats. Cytokine 2011, 56, 739–748.
- Li-Weber, M.; Giaisi, M.; Treiber, M.K.; Krammer, P.H. The anti-inflammatory sesquiterpene lactone parthenolide suppresses IL-4 gene expression in peripheral blood T cells. Eur. J. Immunol. 2002, 32, 3587–3597.
- Humar, M.; García-Piñeres, A.J.; Castro, V.; Merfort, I. Effect of sesquiterpene lactones on the expression of the activation marker CD69 and of IL-2 in T-lymphocytes in whole blood. Biochem. Pharmacol. 2003, 65, 1551–1563.
- Li, S.; Gao, X.; Wu, X.; Cheng, L.; Zhu, L.; Shen, D.; Tong, X. Parthenolide inhibits LPS-induced inflammatory cytokines through the toll-like receptor 4 signal pathway in THP-1 cells. Acta Biochim. Biophys. Sin. 2015, 47, 368–375.
- Feltenstein, M.; Schühly, W.; Warnick, J.; Fischer, N.; Sufka, K. Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear’s foot. Pharmacol. Biochem. Behav. 2004, 79, 299–302.
- Pae, H.-O.; Jeong, G.-S.; Woo, W.H.; Rhew, H.Y.; Kim, H.S.; Sohn, D.H.; Kim, Y.-C.; Chung, H.-T. Costunolide inhibits production of tumor necrosis factor-α and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflamm. Res. 2007, 56, 520–526.
- Sobota, R.; Szwed, M.; Kasza, A.; Bugno, M.; Kordula, T. Parthenolide Inhibits Activation of Signal Transducers and Activators of Transcription (STATs) Induced by Cytokines of the IL-6 Family. Biochem. Biophys. Res. Commun. 2000, 267, 329–333.
- Butturini, E.; Cavalieri, E.; De Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; et al. Two Naturally Occurring Terpenes, Dehydrocostuslactone and Costunolide, Decrease Intracellular GSH Content and Inhibit STAT3 Activation. PLoS ONE 2011, 6, e20174.
- Scarponi, C.; Butturini, E.; Sestito, R.; Madonna, S.; Cavani, A.; Mariotto, S.; Albanesi, C. Inhibition of Inflammatory and Proliferative Responses of Human Keratinocytes Exposed to the Sesquiterpene Lactones Dehydrocostuslactone and Costunolide. PLoS ONE 2014, 9, e107904.
- Kang, J.S.; Yoon, Y.D.; Lee, K.H.; Park, S.-K.; Kim, H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004, 313, 171–177.
- He, Y.; Moqbel, S.A.A.; Xu, L.; Ran, J.; Ma, C.; Xu, K.; Bao, J.; Jiang, L.; Chen, W.; Xiong, Y.; et al. Costunolide inhibits matrix metalloproteinases expression and osteoarthritis via the NF-κB and Wnt/β-catenin signaling pathways. Mol. Med. Rep. 2019, 20, 312–322.
- Saraswati, S.; Alhaider, A.A.; Abdelgadir, A.M. Costunolide suppresses an inflammatory angiogenic response in a subcutaneous murine sponge model. APMIS 2018, 126, 257–266.
- Lee, J.; Tae, N.; Lee, J.J.; Kim, T.; Lee, J.-H. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264.7 cells by inducing proteasomal degradation of TRAF6. Eur. J. Pharmacol. 2010, 636, 173–180.
- Formisano, C.; Sanna, C.; Ballero, M.; Chianese, G.; Sirignano, C.; Rigano, D.; Millán, E.; Muñoz, E.; Taglialatela-Scafati, O. Anti-inflammatory sesquiterpene lactones from Onopordum illyricum L. (Asteraceae), an Italian medicinal plant. Fitoterapia 2017, 116, 61–65.
- Pan, L.; Hu, L.; Zhang, L.; Xu, H.; Chen, Y.; Bian, Q.; Zhu, A.; Wu, H. Deoxyelephantopin decreases the release of inflammatory cytokines in macrophage associated with attenuation of aerobic glycolysis via modulation of PKM2. Int. Immunopharmacol. 2020, 79, 106048.
- Huang, C.-C.; Lin, K.-J.; Cheng, Y.-W.; Hsu, C.-A.; Yang, S.-S.; Shyur, L.-F. Hepatoprotective effect and mechanistic insights of deoxyelephantopin, a phyto-sesquiterpene lactone, against fulminant hepatitis. J. Nutr. Biochem. 2013, 24, 516–530.
- Benteldjoune, M.; Chini, M.G.; Iannuzzi, A.M.; Kabouche, A.; Kabouche, Z.; D’ambola, M.; Marzocco, S.; Autore, G.; Bifulco, G.; De Tommasi, N. Guaianolides from Ormenis mixta: Structural Insights and Evaluation of Their Anti-inflammatory Profile. Planta Med. 2019, 85, 947–956.
- Zhou, Q.; Zhang, W.-X.; He, Z.-Q.; Wu, B.-S.; Shen, Z.-F.; Shang, H.-T.; Chen, T.; Wang, Q.; Chen, Y.-G.; Han, S.-T. The Possible Anti-Inflammatory Effect of Dehydrocostus Lactone on DSS-Induced Colitis in Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 1–8.
- Kim, S.Y.; Heo, S.; Kim, S.H.; Kwon, M.; Sung, N.J.; Ryu, A.-R.; Lee, M.-Y.; Park, S.-A.; Youn, H.-S. Suppressive effects of dehydrocostus lactone on the toll-like receptor signaling pathways. Int. Immunopharmacol. 2020, 78, 106075.
- Sun, Z.; Li, G.; Tong, T.; Chen, J. Micheliolide suppresses LPS-induced neuroinflammatory responses. PLoS ONE 2017, 12, e0186592.
- Viennois, E.; Xiao, B.; Ayyadurai, S.; Wang, L.; Wang, P.G.; Zhang, Q.; Chen, Y.; Merlin, D. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Lab. Investig. 2014, 94, 950–965.
- Qin, X.; Jiang, X.; Wang, Y.; Miao, Z.; He, W.; Yang, G.; Lv, Z.; Yu, Y.; Zheng, Y. Micheliolide inhibits LPS-induced inflammatory response and protects mice from LPS challenge. Sci. Rep. 2016, 6, 23240.
- Xu, H.; Wang, J.; Wang, C.; Chang, G.; Lin, Y.; Zhang, H.; Zhang, H.; Li, Q.; Pang, T. Therapeutic effects of micheliolide on a murine model of rheumatoid arthritis. Mol. Med. Rep. 2014, 11, 489–493.
- Cho, J.Y.; Baik, K.U.; Jung, J.H.; Park, M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000, 398, 399–407.
- Abderrazak, A.; Couchie, D.; Mahmood, D.; El Hage, R.; Vindis, C.; Laffargue, M.; Mateo, V.; Buechele, B.; Ayala, M.R.; El Gaafary, M.; et al. Anti-Inflammatory and Antiatherogenic Effects of the NLRP3 Inflammasome Inhibitor Arglabin in ApoE2.Ki Mice Fed a High-Fat Diet. Circulation 2015, 131, 1061–1070.
- Matos, M.S.; Anastácio, J.D.; Allwood, J.W.; Carregosa, D.; Marques, D.; Sungurtas, J.; McDougall, G.J.; Menezes, R.; Matias, A.A.; Stewart, D.; et al. Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory. Nutrients 2020, 12, 3547.
- Cavin, C.; Delannoy, M.; Malnoe, A.; Debefve, E.; Touché, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005, 327, 742–749.
- Chun, J.; Choi, R.J.; Khan, S.; Lee, D.-S.; Kim, Y.-C.; Nam, Y.-J.; Kim, Y.S. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int. Immunopharmacol. 2012, 14, 375–383.
- Ren, Y.; Yue, B.; Ren, G.; Yu, Z.; Luo, X.; Sun, A.; Zhang, J.; Han, M.; Wang, Z.; Dou, W. Activation of PXR by alantolactone ameliorates DSS-induced experimental colitis via suppressing NF-κB signaling pathway. Sci. Rep. 2019, 9, 16636.
- Liu, L.; Hua, Y.; Wang, D.; Shan, L.; Zhang, Y.; Zhu, J.; Jin, H.; Li, H.; Hu, Z.; Zhang, W. A Sesquiterpene Lactone from a Medicinal Herb Inhibits Proinflammatory Activity of TNF-α by Inhibiting Ubiquitin-Conjugating Enzyme UbcH5. Chem. Biol. 2014, 21, 1341–1350.
- Wang, X.; Tang, S.-A.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Inhibitory Effects of JEUD-38, a New Sesquiterpene Lactone from Inula japonica Thunb, on LPS-Induced iNOS Expression in RAW264.7 Cells. Inflammation 2015, 38, 941–948.
- Fonseca, L.C.; Dadarkar, S.S.; Lobo, A.S.; Mishra, P.B.; Thakkar, A.D.; Chandrababu, S.; Padigaru, M. NF-κB-mediated anti-inflammatory activity of the sesquiterpene lactone 7-hydroxyfrullanolide. Eur. J. Pharmacol. 2011, 657, 41–50.
- Fonseca, L.C.; Dadarkar, S.S.; Lobo, A.S.; Suthar, A.C.; Chauhan, V.S.; Chandrababu, S.; Sharma, S.D.; Dagia, N.M.; Padigaru, M. 7-hydroxyfrullanolide, a sesquiterpene lactone, inhibits pro-inflammatory cytokine production from immune cells and is orally efficacious in animal models of inflammation. Eur. J. Pharmacol. 2010, 644, 220–229.
- Choi, H.-G.; Lee, D.-S.; Li, B.; Choi, Y.H.; Lee, S.-H.; Kim, Y.-C. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012, 13, 271–279.
- Ferrari, F.C.; Ferreira, L.C.; Souza, M.R.; Paula, C.A.; Rezende, S.A.; Grabe-Guimarães, A.; Saúde-Guimarães, D. Anti-Inflammatory Sesquiterpene Lactones from Lychnophora trichocarpha Spreng. (Brazilian Arnica). Phytother. Res. 2012, 27, 384–389.
- Zarpelon, A.C.; Fattori, V.; Souto, F.O.; Pinto, L.G.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Turato, W.M.; Cunha, T.M.; Da Costa, F.B.; Cunha, F.Q.; et al. The Sesquiterpene Lactone, Budlein A, Inhibits Antigen-Induced Arthritis in Mice: Role of NF-κB and Cytokines. Inflammation 2017, 40, 2020–2032.
- Valério, D.A.; Cunha, T.M.; Arakawa, N.S.; Lemos, H.D.P.; Da Costa, F.B.; Parada, C.A.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: Inhibition of cytokine production-dependent mechanism. Eur. J. Pharmacol. 2007, 562, 155–163.
- Fattori, V.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Borghi, S.M.; Zaninelli, T.H.; Da Costa, F.B.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; Casagrande, R.; et al. Budlein A, a Sesquiterpene Lactone from Viguiera robusta, Alleviates Pain and Inflammation in a Model of Acute Gout Arthritis in Mice. Front. Pharmacol. 2018, 9, 1076.
- Sabel, R.; Fronza, A.; Carrenho, L.; Maes, A.; Barros, M.; Pollo, L.A.E.; Biavatti, M.; D’Herde, K.; Vandenabeele, P.; Kreuger, M. Anti-inflammatory activity of the sesquiterpene lactone diacethylpiptocarphol in dextransulfate sodium-induced colitis in mice. J. Ethnopharmacol. 2019, 245, 112186.
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an Anti-Inflammatory Sesquiterpene Lactone from Arnica, Selectively Inhibits Transcription Factor NF-κB. Biol. Chem. 1997, 378, 951–962.
- Lyß, G.; Knorre, A.; Schmidt, T.J.; Pahl, H.L.; Merfort, I. The Anti-inflammatory Sesquiterpene Lactone Helenalin Inhibits the Transcription Factor NF-κB by Directly Targeting p65. J. Biol. Chem. 1998, 273, 33508–33516.
- Berges, C.; Fuchs, D.; Opelz, G.; Daniel, V.; Naujokat, C. Helenalin suppresses essential immune functions of activated CD4+ T cells by multiple mechanisms. Mol. Immunol. 2009, 46, 2892–2901.
- Zwicker, P.; Schultze, N.; Niehs, S.; Albrecht, D.; Methling, K.; Wurster, M.; Wachlin, G.; Lalk, M.; Lindequist, U.; Haertel, B. Differential effects of Helenalin, an anti-inflammatory sesquiterpene lactone, on the proteome, metabolome and the oxidative stress response in several immune cell types. Toxicol. Vitr. 2017, 40, 45–54.
- Lim, C.B.; Fu, P.Y.; Ky, N.; Zhu, H.S.; Feng, X.; Li, J.; Srinivasan, K.G.; Hamza, M.S.; Zhao, Y. NF-κB p65 repression by the sesquiterpene lactone, Helenalin, contributes to the induction of autophagy cell death. BMC Complement. Altern. Med. 2012, 12, 93.
- Gertsch, J.; Sticher, O.; Schmidt, T.; Heilmann, J. Influence of helenanolide-type sesquiterpene lactones on gene transcription profiles in Jurkat T cells and human peripheral blood cells: Anti-inflammatory and cytotoxic effects. Biochem. Pharmacol. 2003, 66, 2141–2153.
- Tornhamre, S.; Schmidt, T.J.; Näsman-Glaser, B.; Ericsson, I.; Lindgren, J. Åke Inhibitory effects of helenalin and related compounds on 5-lipoxygenase and leukotriene C4 synthase in human blood cells. Biochem. Pharmacol. 2001, 62, 903–911.
- Wu, Z.-L.; Wang, J.-X.; Chen, L.-P.; Dong, H.-Y.; Li, H.-L.; Zhang, W.-D. Five rare C 32 sesquiterpene lactone dimers with anti-inflammation activity from Vladimiria souliei. Fitoterapia 2018, 125, 117–122.
- Wang, Y.; Cao, J.; Zhaofang, Y.; Xie, Y.; Xu, Z.; Yin, Z.; Gao, L.; Wang, C. Artemisinin inhibits monocyte adhesion to HUVECs through the NF-κB and MAPK pathways in vitro. Int. J. Mol. Med. 2016, 37, 1567–1575.
- Kim, H.G.; Yang, J.H.; Han, E.H.; Choi, J.H.; Khanal, T.; Jeong, M.H.; Jeong, T.C.; Jeong, H.G. Inhibitory effect of dihydroartemisinin against phorbol ester-induced cyclooxygenase-2 expression in macrophages. Food Chem. Toxicol. 2013, 56, 93–99.
- Siedle, B.; Cisielski, S.; Murillo, R.; Löser, B.; Castro, V.; Klaas, C.; Hucke, O.; Labahn, A.; Melzig, M.; Merfort, I. Sesquiterpene lactones as inhibitors of human neutrophil elastase. Bioorg. Med. Chem. 2002, 10, 2855–2861.




