Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatrice Mihaela Radu | + 4637 word(s) | 4637 | 2021-07-01 05:27:13 | | | |
| 2 | Bruce Ren | -21 word(s) | 4616 | 2021-07-14 03:35:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Radu, B.M. LOCs/OOCs for Biomedical Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/12029 (accessed on 08 February 2026).
Radu BM. LOCs/OOCs for Biomedical Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/12029. Accessed February 08, 2026.
Radu, Beatrice Mihaela. "LOCs/OOCs for Biomedical Applications" Encyclopedia, https://encyclopedia.pub/entry/12029 (accessed February 08, 2026).
Radu, B.M. (2021, July 13). LOCs/OOCs for Biomedical Applications. In Encyclopedia. https://encyclopedia.pub/entry/12029
Radu, Beatrice Mihaela. "LOCs/OOCs for Biomedical Applications." Encyclopedia. Web. 13 July, 2021.
Copy Citation
Lab-on-a-chip (LOC) and organ-on-a-chip (OOC) devices are highly versatile platforms that enable miniaturization and advanced controlled laboratory functions (i.e., microfluidics, advanced optical or electrical recordings, high-throughput screening). The manufacturing advancements of LOCs/OOCs for biomedical applications and their current limitations are briefly discussed. Multiple studies have exploited the advantages of mimicking organs or tissues on a chip. Among these, we focused our attention on the brain-on-a-chip, blood–brain barrier (BBB)-on-a-chip, and neurovascular unit (NVU)-on-a-chip applications.
lab-on-a-chip
organ-on-a-chip
microfluidic platforms
blood brain barrier
neurovascular unit
drug screening
neurodegenerative disorders
1. Introduction
Lab-on-a-chip (LOC) devices are promising microfluidic platforms that allow miniaturization and the integration of multiple laboratory functions. They may accommodate specific components and functions, such as electronics, optics, fluidics, or biosensing structures, at a centimeter/millimeter down to micro- and nanoscale [1][2].
These microdevices are used in different types of laboratory analyses, biochemical operations, DNA sequencing, or chemical synthesis. Among the applications in which LOC platforms can play important roles, one may outline the analysis of ions from different compositions used in fields such as forensics, the identification of explosives, evaluation of water quality, study of body fluids, in agricultural domain or detection of pollution levels [3].
In the last years, the LOC platforms used for biological purposes have been intensely developed, with a special focus on three-dimensional (3D) configurations. While decreasing device sizes, small volumes have significant benefits, which include reduced reagent costs and increased accuracy of analysis. Such biochips, made of glass or polymers, allow biological investigations at the cellular level, including single cell analysis. These 3D in vitro models may represent alternatives for animal sacrifices and in vivo experiments, due to the quasi-realistic reproduction of the physiological systems [4].
Thus, LOCs can be used in different studies targeting organ/tissue models, including the blood–brain barrier, blood vessels, kidney, heart, lung, liver, intestine, muscle, or even tumors [5]. The advantages of this technology rely on increased spatial resolution for interrogation, automated measurements, robustness, low costs, and user-friendly properties [6].
2. LOC Materials and Manufacturing Advancements for Biomedical Research
During the last few decades, microfluidics has triggered various developments in different scientific and technological fields such as disease diagnostics, drugs screening, single-cell analyses, biosensing, analytical chemistry, and micro- and nanofabrication [7][8]. LOC materials are processed by various techniques to develop 3D hollow structures of small dimensions down to the micro- and nanoscale in different complex shapes including channels, chambers, or valves [9].
At the same time asthe broad spectrum of applications diversification, strong advances were achieved in the development of appropriate materials and microfabrication technologies. Briefly, there are six main types of materials currently used for the manufacturing of microchips: silicon/glass, poly(dimethylsiloxane) (PDMS), thermoplastics, thermosets, paper, and more recently, hydrogels [10].
Although the fabrication costs could be high (clean room conditions and/or sophisticated processing equipment are needed), inorganic materials may allow accurate processing with high spatial resolution for microfluidic devices. Organic materials are good alternatives, although they involve multiple technological processing steps, including, casting, molding, replication, bonding, and sometimes limiting usage. Lately, paper microfluidics is focusing on a limited number of applications only, while hydrogels are considered as relevant biomimetic materials for microfluidic assays, which are also suitable for 3D bioprinting. The main physical–chemical properties of these materials and current processing technologies employed for device fabrication are summarized in Table 1.
Table 1. Properties of materials and processing technologies typically used for the fabrication of microfluidic devices (adapted from [11]).
| Material/Property | Silicon/Glass | Elastomers | Thermosets | Thermoplastics | Hydrogel | Paper |
|---|---|---|---|---|---|---|
| optical transparency | no/high | high | high | medium to high | low to medium | low |
| hydrophobicity | hydrophilic | hydrophobic | hydrophobic | hydrophobic | hydrophilic | amphiphilic |
| thermostability | very high | medium | high | medium to high | low | medium |
| resistance to oxidizer | excellent | moderate | good | moderate to good | low | low |
| solvent compatibility | very high | low | high | medium to high | low | medium |
| permeability to oxygen (Barrer a) | <0.01 | ≈500 | 0.03–1 | 0.05–5 | >1 | >1 |
| surface charge | very stable | not stable | stable | stable | N/A | N/A |
| common technique for microfabrication/features | photolithography, laser-assisted etching | casting | casting, photopolymerization | thermo-molding | casting, photopolymerization, 3D bioprinting | photolithography, printing |
| smallest channel dimension | <100 nm | <1 μm | <100 nm | ≈100 nm | ≈10 μm | ≈200 μm |
| channel profile | limited 3D/3D | 3D | arbitrary 3D | 3D | 3D | 2D |
| multilayer channels | hard/easy | easy | easy | easy | Medium | easy |
| throughput | medium to high | high | high | high | low to medium | high |
Silicon and glass were the first materials used to develop LOC platforms [12]. The technologies used in the fabrication of microfluidic biochips have expanded during the last few years [13]. Since silicon is expensive and optically opaque in the visible spectrum, there are some limitations for its biological use. Polymers appeared as a relevant alternative and contributed to a rapid advancement of the microfluidics field. Then, the non-photolithographic micro- and nanofabrication of micro-systems was possible in regular laboratory rooms, without the need for clean room equipment. This involved using elastomeric stamps to create patterns with feature sizes down to a few tens of nanometers [14]. Microfluidic systems made of PDMS, an optically transparent soft elastomer, were then the most employed structures with characteristics exploited to control various patterns and microchannels relevant to biology for cellular studies [1]. We further present PDMS and glass-based LOCs as they may offer a good trade-off between flexibility to be processed, transparency, biocompatibility, range of applications, and costs.
2.1. PDMS LOCs
PDMS is the most used material in microfluidics for LOC applications due to its relative facile fabrication and relevant properties such as resistance at chemical, physical, or biological agents [15]. The PDMS material confers a number of advantages: it is biocompatible, cheap, easy to model, transparent, and facilitates biological studies on cell cultures due to its properties regarding gas and water permeability [9][16][17][18][19][20][21][22].
PDMS surfaces are rather hydrophobic, but they may become hydrophilic by oxygen plasma treatment [23], modification using oxygen and C2F6, using oxygen plasma polymerization of 2-hydroxyethyl methacrylate (HEMA) [24], using atmospheric RF plasma [25], by oxygen plasma treatment, followed by treatment in deionized water [26], corona/air plasma [27], or even surface treatment with NaOH, especially when it comes to microchannels [28]. Indeed, the main drawback of PDMS, in particular for biomedical applications, resides in its hydrophobic properties (poor surface wetting and heterogeneous charge), which may further induce the undesired adsorption of organic molecules. On the other hand, there are several approaches that addressed hydrophilicity conservation by combining UV irradiation and oxygen plasma [29] or chemical grafting treatments [30][31]; that may increase surface wetting stability from tens of minutes up to six months. Simple alternatives for surface hydrophilicity conservation also include the storing of PDMS under water [32] or at very low temperatures [33] after oxygen-based plasma treatment. All these aspects must be carefully addressed in correlation with the required channel geometry for the envisaged application.
The PDMS LOCs are intensively used in either 2D or 3D configurations [34]. They are relatively easy to be manufactured by lithographic techniques in rather short times and at minimal costs [35][36]. Specifically, the process of a chip production by photolithography consists of a mold fabrication with a desired geometrical configuration chosen for a specific application. The mold can be obtained by the direct light irradiation of a photoresist followed by chemical development of the material to obtain the desired design and subsequent PDMS casting to create the microfluidic chips [35] (schematic example shown in Figure 1).Although the process is rather laborious and time consuming due to several technological steps, the mold could be reused for the replication of several biochips with high accuracy. Two-photon polymerization (TPP) is a laser lithographic technology that employs ultrashort pulsed lasers to fabricate polymeric structures with nanoscale resolution. The polymerization is initiated by a laser beam focused through an objective onto a photoresist material. When applied to negative-tone photoresists, TPP is considered an additive processing technique because the polymerization occurs throughout the scanning trajectory of the focused laser beam while non-exposed areas are washed away by solvents. Theoretically, there is no limitation of resolution due to the material threshold effect correlated with the precision control of the high peak laser intensity, so that sub-100 nm features can be obtained [37]. However, this technique may not be appropriate for large area processing but rather for downsizing dimensions in microfluidic platforms.
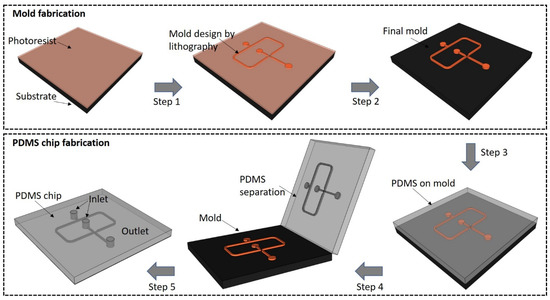
Figure 1. Schematic representation of a droplet generator microchip based on the technology used in our laboratory: ultrashort pulsed laser lithography applied for the mold fabrication (steps 1 and 2) followed by PDMS casting (steps 3–5).
A limitation in the manufacture of these microdevices arises when true 3D structures are desired, since more than one mold is needed. Therefore, several attempts have been employed to obtain chips with 3D microenvironments [36]. One approach is to create individual 2D structures that are further interconnected and bonded together using air plasma [38] or oxygen plasma [39][40][41]. Although successful, this method is time consuming and requires high precision in the construction and alignment of the parts, which could be a strong drawback. Another approach is to use the new 3D printing technologies for direct writing of the structures without requiring too much intervention of the users. However, the equipment is still expensive, and the dyes used in the printing may render the polymer opaque, thus limiting the optical interrogation [36].
2.2. Glass LOCs
Due to high chemical and temperature resistance, inertness to many substances, and low nonspecific adsorption, glass materials are of great interest for microfluidic applications in biology [42]. Glass exhibits a high degree of transparency and can be rather easy to be processed either by chemical or physical methods.The use of glass in the manufacturing processes of microfluidic devices offers some important advantages over polymeric materials, such as robustness, higher optical quality, or low adsorption of organic compounds. Wet or dry chemical etching techniques or mechanical processes can be applied for the fabrication of glass micro-scale devices but with low precision and productivity as compared to lithographic processes [42]. The use of lasers in combination with a liquid environment allows glass machining with better control over heat and crack. Depending on the final application, glasses such as quartz, borosilicate glass (Pyrex) [43], soda lime glass [44], or photosensitive glass can be used to manufacture both 2D and 3D free-form microfluidic devices, usually through a laser irradiation process, followed by wet or dry etching, in which the exposed region is removed with high selectivity. Glasses can be also bonded with PDMS to form complex 3D structures or microfluidic connections. This bonding is achieved by various methods such as oxygen plasma treatment [45], oxygen plasma followed by heat treatment [46], air plasma/corona [47], or using chemical crosslinking agents [48]. These glass–polymer hybrid structures can be used for specific biology studies, with increased capabilities of reproducing physiological environments [49]. Photosensitive glasses are a category of glasses that allow microfabrication by UV or laser irradiation followed by etching for microfluidic applications. Femtosecond and picosecond laser-assisted etching are subtractive 3D processing methods that use laser direct writing, thermal treatment, and subsequent chemical wet etching to fabricate true 3D hollow channels inside glass (Figure 2) [50][51].

Figure 2. Schematic of photosensitive glass processing based on the technology used in our laboratory: (a) picosecond laser direct writing (LDW); (b) thermal annealing and (c) selective glass etching.
Thus, it is then possible to fabricate complex, 3D channels in glass for specific biomicrofluidic applications [52][53]. By laser technologies, one may create microfluidic circuits even on large areas, without supplementary steps of stacking or bonding while specific properties of glass such as robustness, portability, and transparency are preserved. Such glasses are biocompatible, easy to clean, and consequently reusable as 3D biochips or even molding systems [51][54]. A heat treatment can be applied to these materials to obtain a very smooth surface necessary to create relevant cellular environments [55][56].
On the other hand, hybrid subtractive and additive processing can be combined to develop functional polymeric structures inside robust, highly transparent glass microchannels. Specifically, subtractive laser etching of glass followed by the additive polymerization of negative photoresists can be applied to fabricate polymeric 3D microstructures inside embedded glass microfluidic channels [57]. Thus, one may downsize, below 1 μm, dimensions of various 3D complex objects while improving the structure stability [56]. This process allows users to customize complex designs to obtain reliable 3D biochips for concrete applications.
2.3. Biomedical Applications of LOCs
It is common knowledge that 2D cell culture and animal models exhibit limited predictability for drug discovery, and therefore, there is an urgent need to find better models for efficient and reproducible drug screening. On the other hand, the ethical rules governing the experiments involving laboratory animals became more and more restrictive, limiting the in vivo preclinical analysis extent. In this context, LOC platforms seem to be a robust technology with extended customization possibilities that can replace the standard cell cultures and animal models in biomedical approaches. Organ on-a-chip (OOC) is a well-established transdisciplinary technology that is facing challenges at aiming to develop microfluidic-based perfusion devices able to mimic the keyfunctions of a specific organ/tissue in both normal and pathological microphysiology [58].
To date, several organs and tissues have been mimicked on a chip (see Table 2), including alveolus [59], bone marrow [60], gut [61], heart [62][63], lung [64], pancreas [65], skin [66], or complex interactions between tissues have been integrated on a chip, such as lung–liver–heart [67] or intestine–liver–brain–kidney [68]. Such OOC applications are targeting the analysis of the cellular behavior in physiological conditions, the development of screening platforms to test the cellular response to various drugs or stimuli, or the in vitro modeling of a pathological condition (e.g., inflammation, edema etc.).
Table 2. Organ-on-a-chip applications.
| Organ/Tissue Type | Chip Material | Membrane Material | Application | Reference |
|---|---|---|---|---|
| Alveolus-on-a-chip | PDMS | PDMS | Interface alveolar epithelium/endothelium for the study of inflammation-induced thrombosis | [59] |
| Bone marrow-on-a-chip | PDMS | PDMS | Analysis of the cellular response to drugs and radiation | [60] |
| Gut-on-a-chip | PDMS | Polyester | Development of a platform for drug screening and substance toxicity testing | [61] |
| Heart-on-a-chip | PDMS | No membrane | Testing the inotropic effect of isoproterenol on cardiac contractility | [62] |
| PMMA and PDMS | No membrane | Evaluation of cardiovascular toxicity of some pharmaceutical products | [63] | |
| Intestine–liver–brain–kidney-on-a-chip | PDMS | PDMS | Production and testing of an autologous iPSC derived four-organ-on-a-chip in long-term cocultivation conditions (i.e., 14 days) | [68] |
| Kidney-on-a-chip | PDMS | Polyester | Analysis in conditions close to the physiological ones of renal tubule cells | [69] |
| Lung-on-a-chip | PDMS | PDMS | Mimicking and analyzing the long alveolar barrier | [64] |
| Lung–liver–heart-on-a-chip | PMMA and PDMS | Polyester | Assessment of the importance of interactions between organs in response to drugs | [67] |
| Pancreas-on-a-chip | PDMS | Polyester | Investigating the role of CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) in insulin production | [65] |
| Skin-on-a-chip | PDMS | Polyester | Mimicking edema and inflammation of the skin and testing dexamethasone effects | [66] |
A comprehensive review devoted to recent advances in the field of organ-on-a-chip engineering was reported by Zhang et al. [34]. The authors discuss how OOC technology can mimic the keyfunctions of organs, in close relation with human physiology, by focusing on tissue barrier properties, parenchymal tissue function, and multi-organ interactions. In a different study, Maschmeyer et al. developed a four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin, and kidney equivalents [70], and they showed the preservation of the microphysiological functionality of the system over 28 days. In a critical review, Junaid et al. advanced an end-user perspective on the latestOOC developments and highlighted how the validated academic proof-of-concept studies could be translated to real-world societal solutions [71]. The challenges for bridging the gap between lab and industry in the field of OOC technologies were recently addressed by Ramadan and Zourob [58]. OOC is a well-recognized multidisciplinary approach that is expected to change many aspects of preclinical-to-clinical translation in the biomedical field. However, there are still many scientific and technical challenges, as well as standardization and regulatory endorsement that should be overcome before technological transfer and commercialization of OOC microdevices.
In the last years, several studies have focused their attention on employing the OOC technology to obtain brain-on-a-chip devices. An integrative review describes the strategies of fabrication for brain-on-a-chip devices and their relevance/compliance as testing platforms for pharmacological screening and disease monitoring [72]. Miccoli et al. emphasized the impact of exploiting OOC platforms, instead of animal models, to perform preclinical pharmacological tests and highlighted the importance of using patient-derived neurons for a strong model reliability [72]. Indeed, the best way to achieve a good 2D or 3D microfluidic brain-on-a-chip model is to combine the use human stem cells (e.g., neural stem cells, induced pluripotent stem cells or embryonic stem cells) with advantages of such a device, including the use of a small amount of fluid, the possibility of creating shear stress conditions, and the low costs of production [73]. Further technological developments employed in brain-on-a-chip devices, such as optogenetics, brain organoids, and 3D bioprinting, are also essential, taking into account the challenge of integrating the complexity of neuronal architectures and connectivity (i.e., 52 regions with distinct cellular organization in human brain) on a chip mimicking brain physiology and pathology [74][75].
A collection of studies devoted to brain-on-a-chip models is presented in Table 3. The majority of the brain-on-a-chip models are based on organoids/neurospheroids obtained either from human stem cells or primary rodent neuronal cultures [41][76]. In detail, brain-on-a-chip devices have been used to model neurodevelopmental disorders due to prenatal nicotine exposure [76], neurodegenerative disorders [41][77], neural transplantation therapy in severe degenerative brain diseases [78], amyloid-β induced axonopathy [79], etc. More insights on BBB- and NVU-on-a-chip are presented in the next section of this review.
Table 3. PDMS microfluidicbrain-on-a-chip platforms.
| Organ/Tissue Type | Type of Cells | Application | Reference |
|---|---|---|---|
| Brain organoid-on-a-chip | 3D brain organoids derived from human-induced pluripotent stem cells (hiPSCs) | Modeling the neurodevelopmental disorders under environmental exposure (e.g., nicotine) | [76] |
| 3D brain-on-a-chip | Neurospheroids obtained from prenatal E16 rat cortical neurons | In vitro brain model for neurodegenerative disease (e.g., Alzheimers’ disease) and high-throughput drug screening | [41] |
| Brain-on-a-chip | Neurospheroids obtained from human neural progenitor and human iPSC-derived neural progenitor cells | Investigating the development of Alzheimer’s disease and testing drugs against this neuropathology | [77] |
| Neurospheroid network-on-a-chip | Neurospheroids obtained from primary culture obtained from the cerebral cortex of Wistar rats | Studying neural transplantation therapy for treating severe degenerative brain disease | [78] |
| 3D brain-on-a-chip | Neurospheroids obtained from prenatal rat (E18) cortical neurons | Modulation of cell–ECM interactions at the neuronal level by analyzing neurospheroids and their study in pathological conditions | [79] |
3. BBB andNVU on a Chip
The brain is an organ with an extremely sophisticated structure, which requires a large amount of energy that is mainly supplied by blood with the necessary energy substrates (e.g., glucose and oxygen) [80]. In addition, blood transports multiple substances, among which are also waste products (i.e., neurotoxins), whose access inside brain parenchyma should be prevented. In this context, the brain capillaries’ walls form an interface, called the BBB, with a set of structural and functional features that regulate the transport of substances from the blood to the brain and the other way around [81]. This barrier is largely composed of specialized brain microvascular endothelial cells that separate blood from the interstitial fluids of the brain and, together with the choroid plexus and the arachnoid, help maintain brain homeostasis. The BBB also mediates the passive and active transport of the elements, and it plays an important role as an immunological and metabolic barrier [82].
The BBB is part of the NVU, along with neurons, astrocytes, pericytes, microglia, and the extracellular matrix [83][84][85]. To define, the NVU is considered a set of structures that allow the coordinated response between brain parenchyma and cerebral vascular endothelium to be maintained [86]. Neurons are responsible for using/detecting oxygen and nutrient changes and transforming this information into electrical or chemical signals, which they send to astrocytes either directly or through interneurons creating communication networks [85]. Astrocytes, which are five times more abundant than neurons, are important actors of NVU that regulate cerebral blood flow and brain energy metabolism, or are partners in gliotransmission [86][87][88]. Pericytes also play an important role in the NVU, being in direct contact with the brain endothelial cells, offering them support and actively participating in their development and maturation [85]. Microglia are immunocompetent cells of the NVU, acting as pathological sensors, whose activity is to constantly investigate the intracranial environment, and to remove the damaged cells from dysfunctional synapses or any other debris from brain parenchyma [89].
LOC technology has been intensively applied in recent years as BBB-on-a-chip or NVU-on-a-chip technologies (Figure 3).
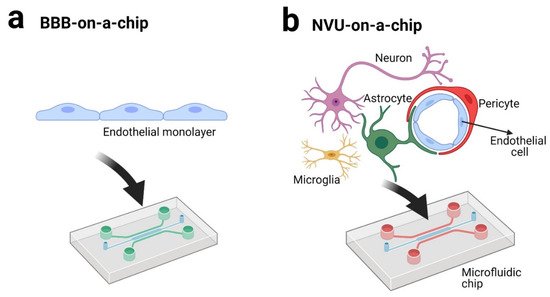
Figure 3. BBB-on-a-chip (a) and NVU-on-a-chip (b) technologies (created with BioRender.com [90]).
These technologies have been employed for studying the following (Table 4): the role of BBB in neuroinflammatory, neurodegenerative (e.g., Alzheimer’s, Parkinson’s), or in schizophrenia pathologies [19][21][73][91][92], the interactions between BBB and combinations of cytokines and lipopolysaccharides, leading to loss of function [93][94], the permeability of BBB for drugs or endogenous molecules [16][22][95], the biochemical modulation of BBB [96], the antibody interaction with BBB [22][97], the neuronal–endothelial metabolic coupling [17], or the interaction between cancer cells and astrocytes in a BBB microenvironment [98].
Table 4. BBB-on-a-chip and NVU-on-a-chip applications.
| Model | Chip Material | Membrane Material | Culture Type | Cells | Application | Reference |
|---|---|---|---|---|---|---|
| BBB | PDMS and glass | Polycarbonate | Co-culture | Endothelial cells (b.End3) and astrocytes (C8D1A) | BBB permeability | [99] |
| BBB | PDMS | Polyethylene terephthalate | Co-culture | Endothelial cells (BMEC from hiPCS) and astrocytes (from IMR90-4 iPSCs) | BBB permeability due to TNF-α in liver failure/melanoma | [100] |
| BBB | OrganoPlate | No membrane | Tri-culture | Endothelial cells (TY10), astrocytes (hAst) and pericytes (hBPCT) | BBB permeability for different types of molecules (antibodies) | [97] |
| BBB | Objet Vero Clear, silicone, and PDMS | Polycarbonate | Co-culture | Endothelial cells (BMEC from iPSC) and astrocytes (Rat primary culture) | BBB permeability for drugs | [101] |
| BBB | PDMS | Polycarbonate | Co-culture | Primary mouse brain microvascular endothelial cells and primary mouse astrocytes | Cellular interactions in the BBB under physiological or shear stress conditions | [102] |
| BBB | PDMS | Polyester and polytetrafluoroethylene | Co-culture | Endothelial cells (b.End3) and astrocytes (C8D1A) | Analysis of cell cultures on porous membranes | [103] |
| BBB | PMMA | Polyester | Monoculture | Endothelial cells (b.End3) | Transport of nanoparticles across the BBB | [104] |
| BBB | PDMS and polyvinylidene fluoride (PVDF) | Polyvinylidene fluoride (PVDF) | Co-culture | Human cerebral microvascular endothelial cells (hCMEC/D3) and normal human astrocytes | Reproducible platform for the BBB study under static or continuous flow conditions | [105] |
| BBB | PDMS | Polycarbonate | Tri-culture | Human cerebral microvascular endothelial cells (HBMEC), pericytes, and astrocytes | BBB model for the investigation of neuroinflammation | [106] |
| BBB | PDMS | No membrane | Multi-Culture | Endothelial cells (HBMEC and HUVEC), pericytes (HhPC-PL), astrocytes (NHA), and primary normal human lung fibroblasts (LF) | In vitro reproduction of angiogenesis in the central nervous system | [107] |
| BBB | PDMS, PMMA, and PC | N/A | Co-culture | Endothelial cells (HUVEC) and human astrocytes | Testing the biocompatibility of the APTES-coated PDMS surface, on which different types of coating were applied | [108] |
| NVU | PDMS | No membrane | Tri-culture | Human iPSC-derived blood–brain barrier cells Human primary astrocytes Human primary pericytes |
Complex platform for the study of neurological diseases | [109] |
| NVU | PDMS | PDMS | Co-culture (×2) | Human teratocarcinoma NTERA-2 cl. D1 (hNT2) cells and human endothelial cells (hBMEC) Human teratocarcinoma NTERA-2 cl. D1 (hNT2) cells and Human fetal neural progenitor cells (hNPCs) |
Differentiation of cells on the chip and analysis of the importance of cell interactions in neurodevelopment | [110] |
| NVU | PDMS | No membrane | Multi-Culture | Endothelial cells (HUVEC and hCMEC/D3), neurons (primary culture), and astrocytes (primary culture) | Neurovascular unit development | [16] |
| NVU | PDMS andpolycarbonate | Polyethylene terephthalate andpolycarbonate | Multi-Culture | Human hippocampal neural stem cells HIP-009 cells, cortical human brain microvascular endothelial cells (hBMVECs), human astrocytes, and human brain pericytes of cortical origin | Effect of intravascular administration of methamphetamine | [17] |
Some of the recent LOC studies devoted to blood–brain barrier summarizing cell sources, functional hallmarks, disease models, and drug tests were reviewed by [34]. Booth and Kim [99], developed a microfluidic blood–brain barrier (µBBB) in order to mimic the dynamic in vivo microenvironment and a comparatively thin culture membrane of 10 µm. The authors proved the validity of the model using co-cultures of bEnd.3 endothelial cells and C8-D1A astrocytes, and they concluded that such system can be used to predict the rate of delivery of new drugs across the BBB, being a valid option for preclinical studies. The research team of Prabhakarpandian et al. demonstrated the similarity between the Synthetic Microvasculature model of BBB (SyM-BBB) and the cerebral microvascularization observed in vivo. They used a microfluidic chip made of PDMS connected to a perfusion system, and as cells, they used rat brain endothelial cell line (RBE4). They performed different tests on cells grown on the surface of the fabricated microdevices; the cultured cells were subjected to astrocyte conditioned media, in the infusion system, for 96 h. Following comparative tests between transwell chambers using porous membranes and SyM-BBB, fluorescence type, Western blot, efflux transporter studies, etc., they concluded that the cells behave on a chip similar to the functional cells in vivo from BBB [111]. Additionally, Jeong et al. tested a 3D arrayed microfluidic BBB-on-a-chip model and integrated an electrical sensor to measure the transendothelial electrical resistance (TEER), and they concluded that their chip mimicked closely the in vivo BBB environment [102].
An important aspect that deserves attention is the advantages offered by the BBB-on-a-chip devices in comparison with the traditional transwell system (Table 5). In a study using co-cultures of endothelial cells and astrocytes, Deosarkar et al. demonstrated that the 3D microfluidic platform mimicked the neonatal physiological environment more accurately than the transwell system [112]. Other studies have demonstrated higher resistance values in TEER measurements for the BBB-on-a-chip model compared to the transwell model for brain microvascular endothelial cells with or without astrocytes or pericytes in co-culture [22][94][96][99].These results were similar irrespective of the brain endothelial cells origin (i.e., human or rodent) and demonstrate the superior qualities provided by the BBB-on-a-chip model in comparison to the traditional transwell model in terms of barrier permeability or tight junctions proteins immunostaining.
Table 5. Comparison between the BBB-on-a-chip and BBB in the transwell system.
| Type of Analysis | Comparison | Type of Cells | References |
|---|---|---|---|
| TEER ZO1 immunostaining |
Slightly higher resistance values upon 7 days in culture for BBB-on-a-chip compared to the transwell system Similar ZO1 immunostaining |
human endothelial cells hCMEC/D3 | [96] |
| TEER ZO1 immunostaining |
Astrocyte conditioned medium improves the resistance values of BBB-on-a-chip BBB-on-a-chip has higher resistance values than the transwell model |
Rat brain endothelial cells (RBEC) isolated from neonatal rats neonatal rat astrocytes |
[112] |
| TEER | μBBB had significantly higher (10-fold) resistance values than the transwell model for co-cultures | b.End3 endothelial cells, with and without co-cultured C8-D1A astrocytes | [99] |
| Barrier permeability and cytokine release profile | Similar permeability of the human 3D BBB-on-a-chip compared to the non-human cells BBB models or to the inflammatory stimulated models (depending on the presence of astrocytes or pericytes) Significantly higher permeability of the human 3D BBB-on-a-chip compared to co-cultures in static transwell plates |
Co-culture of human brain microvascular endothelial cells, human brain pericytes, human astrocytes (from cortex) | [94] |
| P-glycoprotein (P-gp) permeability | BBB-on-a-chip model, but not the transwell model, enable the study of P-gp efflux pump permeability and its pharmacological blockade (e.g., verapamil) | Human iPS cell line IMR90-4 | [22] |
In comparison to the BBB-on-a-chip model, the NVU-on-a-chip model is more elaborate and requires the use of different types of brain cells (e.g., astrocytes, pericytes, neurons etc.) beside the brain microvascular endothelial cells. Additionally, the calcium signaling machinery of the brain microvascular endothelial cells [113] is strongly influenced by the absence/presence of the NVU adjacent cells. Moreover, the NVU-on-a-chip model implies a complex design with multiple compartments that mimic the blood, brain parenchyma, and cerebral spinal fluid [114]. Several groups have developed NVU-on-a-chip models with innovative architectures. In detail, the different NVU-on-a-chip models were employed to study the neurodevelopment [16][110], the metabolic consequences of inflammatory disruption of the BBB [93], or neurological disorders [109].
An important advantage of the BBB/NVU-on-a-chip is their usefulness in avoiding animal sacrifice for the purpose of these studies. In the future, researchers will try to obtain models on microfluidic chips that are as realistic as possible and perform experiments that mimic the intracranial physiological environment. Although animal models have the great advantage of an intact BBB/NVU with the whole complexity of the brain, there are also major disadvantages including costs, long-term care of animals, and ethical issues [115]. Therefore, despite the obvious limitations, the majority of BBB/NVU functions can be mimicked on a chip, with minimal manufacturing expenses [116], in a variety of configurations and with extended possibilities for drug screening. The progress done so far in using LOC devices as pharmacological screening tools, in particular in neurodegenerative diseases, are detailed in the next sections of this review.
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373.
- Gupta, S.; Ramesh, K.; Ahmed, S.; Kakkar, V. Lab-on-chip technology: Are view on design trends and future scope in biomedical applications. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 311–322.
- Gardeniers, J.; Van den Berg, A. Lab-on-a-chip systems for biomedical and environmental monitoring. Anal. Bioanal. Chem. 2004, 378, 1700–1703.
- Azizipour, N.; Avazpour, R.; Rosenzweig, D.H.; Sawan, M.; Ajji, A. Evolution of biochip technology: Are view fromlab-on-a-chip to organ-on-a-chip. Micromachines 2020, 11, 599.
- Polini, A.; Prodanov, L.; Bhise, N.S.; Manoharan, V.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip: A new tool fordrug discovery. Expert Opin. Drug Discov. 2014, 9, 335–352.
- Ghallab, Y.H.; Badawy, W. Lab-on-a-Chip: Techniques, Circuits, and Biomedical Applications; ArtechHouse: Norwood, MA, USA, 2010.
- Hou, X.; Zhang, Y.S.; Trujillo-de Santiago, G.; Alvarez, M.M.; Ribas, J.; Jonas, S.J.; Weiss, P.S.; Andrews, A.M.; Aizenberg, J.; Khademhosseini, A. Interplay between materials and microfluidics. Nat. Rev. Mater. 2017, 2, 1–15.
- Dong, R.; Liu, Y.; Mou, L.; Deng, J.; Jiang, X. Microfluidics-based biomaterials and biodevices. Adv. Mater. 2019, 31, 1805033.
- Jiang, L.; Li, S.; Zheng, J.; Li, Y.; Huang, H. Recent progress in microfluidic models of the blood-brain barrier. Micromachines 2019, 10, 375.
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal. Chem. 2019, 92, 150–168.
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406.
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993, 261, 895–897.
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60.
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373.
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293.
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip 2017, 17, 448–459.
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874.
- Ning, R.; Zhuang, Q.; Lin, J.-M. Biomaterial-based microfluidics for cell culture and analysis. In Cell Analysis on Microfluidics; Springer: Singapore, 2018; pp. 181–224.
- Zilberzwige-Tal, S.; Gazit, E. Go with the flow—Microfluidics approaches for amyloid research. Chem. Asian J. 2018, 13, 3437–3447.
- Liu, X.; Zheng, W.; Jiang, X. Cell-based assays on microfluidics for drug screening. ACS Sens. 2019, 4, 1465–1475.
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in microfluidicblood–brain barrier (BBB) models. Trends Biotechnol. 2019, 37, 1295–1314.
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M. Hypoxia-enhanced blood-brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 1–12.
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuators B Chem. 2006, 114, 170–172.
- Bodas, D.; Khan-Malek, C. Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron. Eng. 2006, 83, 1277–1279.
- Hong, S.M.; Kim, S.H.; Kim, J.H.; Hwang, H.I. Hydrophilic surface modification of PDMS using atmospheric RF plasma. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2006; p. 108.
- Tan, S.H.; Nguyen, N.-T.; Chua, Y.C.; Kang, T.G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 2010, 4, 032204.
- Hillborg, H.; Sandelin, M.; Gedde, U.W. Hydrophobic recovery of polydimethylsiloxane after exposure to partial discharges as a function of crosslink density. Polymer 2001, 42, 7349–7362.
- Hoek, I.; Tho, F.; Arnold, W.M. Sodium hydroxide treatment of PDMS based microfluidic devices. Lab Chip 2010, 10, 2283–2285.
- De Menezes Atayde, C.; Doi, I. Highly stable hydrophilic surfaces of PDMS thin layer obtained by UV radiation and oxygen plasma treatments. Phys. Status Solidi C 2010, 7, 189–192.
- Lee, D.; Yang, S. Surface modification of PDMS by atmospheric-pressure plasma-enhanced chemical vapor deposition and analysis of long-lasting surface hydrophilicity. Sens. Actuators B Chem. 2012, 162, 425–434.
- Hemmilä, S.; Cauich-Rodríguez, J.V.; Kreutzer, J.; Kallio, P. Rapid, simple, and cost-effective treatments to achieve long-term hydrophilic PDMS surfaces. Appl. Surf. Sci. 2012, 258, 9864–9875.
- Gaš, B.; Zuska, J.; Coufal, P.; van de Goor, T. Optimization of the high-frequency contactless conductivity detector for capillary electrophoresis. Electrophoresis 2002, 23, 3520–3527.
- Jahangiri, F.; Hakala, T.; Jokinen, V. Long-term hydrophilization of polydimethylsiloxane (PDMS) for capillary filling microfluidic chips. Microfluid. Nanofluidics 2020, 24, 1–11.
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advancesinorgan-on-a-chipengineering. Nat. Rev. Mater. 2018, 3, 257–278.
- Morarka, A.; Agrawal, S.; Kale, S.; Kale, A.; Ogale, S.; Paknikar, K.; Bodas, D. Quantum dot based immunosensor using 3D circular microchannels fabricated in PDMS. Biosens. Bioelectron. 2011, 26, 3050–3053.
- Chan, H.N.; Chen, Y.; Shu, Y.; Chen, Y.; Tian, Q.; Wu, H. Direct, one-step molding of 3D-printed structures for convenient fabrication of truly 3D PDMS microfluidic chips. Microfluid. Nanofluidics 2015, 19, 9–18.
- Sugioka, K.; Cheng, Y. Ultra fast lasers—Reliable tools for advanced materials processing. Light Sci. Appl. 2014, 3, e149.
- Takano, A.; Ogawa, T.; Tanaka, M.; Futai, N. On-chip incubation system for long-term microfluidic cell culture. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 8404–8407.
- Jo, B.-H.; VanLerberghe, L.M.; Motsegood, K.M.; Beebe, D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000, 9, 76–81.
- Fleger, M.; Neyer, A. PDMS microfluidic chip with integrated wave guides for optical detection. Microelectron. Eng. 2006, 83, 1291–1293.
- Park, J.; Lee, B.K.; Jeong, G.S.; Hyun, J.K.; Lee, C.J.; Lee, S.-H. Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip 2015, 15, 141–150.
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel fabrication on glass materials for microfluidic devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495.
- Matsuo, S.; Sumi, H.; Kiyama, S.; Tomita, T.; Hashimoto, S. Femtosecond laser-assisted etching of Pyrex glass with aqueoussolution of KOH. Appl. Surf. Sci. 2009, 255, 9758–9760.
- Chang, T.-L.; Chen, Z.-C.; Lee, Y.-W.; Li, Y.-H.; Wang, C.-P. Ultrafast laser ablation of soda-lime glass for fabricating microfluidic pillar array channels. Microelectron. Eng. 2016, 158, 95–101.
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly (dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597.
- Plecis, A.; Chen, Y. Fabrication of microfluidic devices based on glass—PDMS—Glass technology. Microelectron. Eng. 2007, 84, 1265–1269.
- Haubert, K.; Drier, T.; Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab Chip 2006, 6, 1548–1549.
- Aran, K.; Sasso, L.A.; Kamdar, N.; Zahn, J.D. Irreversible, direct bonding of nanoporous polymer membranes to PDMS or glass microdevices. Lab Chip 2010, 10, 548–552.
- Sima, F.; Xu, J.; Wu, D.; Sugioka, K. Ultrafast laser fabrication of functional biochips: New avenues for exploring 3D micro-and nano-environments. Micromachines 2017, 8, 40.
- Sugioka, K.; Cheng, Y. Femtosecond laser processing for optofluidic fabrication. Lab Chip 2012, 12, 3576–3589.
- Jipa, F.; Iosub, S.; Calin, B.; Axente, E.; Sima, F.; Sugioka, K. High repetition rate UV versus VIS picosecond laser fabricationof 3D microfluidic channels embedded in photosensitive glass. Nanomaterials 2018, 8, 583.
- Hanada, Y.; Sugioka, K.; Shihira-Ishikawa, I.; Kawano, H.; Miyawaki, A.; Midorikawa, K. 3D microfluidic chips with integrated functional microelements fabricated by a femtosecond laser for studying the gliding mechanism of cyanobacteria. Lab Chip 2011, 11, 2109–2115.
- Sima, F.; Sugioka, K.; Vázquez, R.M.; Osellame, R.; Kelemen, L.; Ormos, P. Three-dimensional femtosecond laser processing for lab-on-a-chip applications. Nanophotonics 2018, 7, 613–634.
- Jipa, F.; Orobeti, S.; Butnaru, C.; Zamfirescu, M.; Axente, E.; Sima, F.; Sugioka, K. Picosecond Laser Processing of Photosensitive Glass for Generation of Biologically Relevant Microenvironments. Appl. Sci. 2020, 10, 8947.
- Sima, F.; Kawano, H.; Hirano, M.; Miyawaki, A.; Obata, K.; Serien, D.; Sugioka, K. Mimicking intravasation–extravasation with a3D glass nanofluidic model for the chemotaxis-free migration of cancer cells in confined spaces. Adv. Mater. Technol. 2020, 5, 2000484.
- Sima, F.; Kawano, H.; Miyawaki, A.; Kelemen, L.; Ormos, P.; Wu, D.; Xu, J.; Midorikawa, K.; Sugioka, K. 3D biomimetic chips for cancer cell migration in nanometer-sized spaces using “Ship-in-a-Bottle” femtosecond laser processing. ACS Appl. Biomater. 2018, 1, 1667–1676.
- Wu, D.; Wu, S.Z.; Xu, J.; Niu, L.G.; Midorikawa, K.; Sugioka, K. Hybrid femtosecond laser microfabrication to achieve true 3D glass/polymer composite biochips with multiscale features and high performance: The concept of ship-in-a-bottle biochip. Laser Photonics Rev. 2014, 8, 458–467.
- Ramadan, Q.; Zourob, M. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 2020, 14, 041501.
- Jain, A.; Barrile, R.; van der Meer, A.D.; Mammoto, A.; Mammoto, T.; DeCeunynck, K.; Aisiku, O.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A. Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharmacol. Ther. 2018, 103, 332–340.
- Torisawa, Y.-S.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11, 663–669.
- Kimura, H.; Yamamoto, T.; Sakai, H.; Sakai, Y.; Fujii, T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip 2008, 8, 741–746.
- Agarwal, A.; Goss, J.A.; Cho, A.; McCain, M.L.; Parker, K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13, 3599–3608.
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59.
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015, 15, 1302–1310.
- Mun, K.S.; Arora, K.; Huang, Y.; Yang, F.; Yarlagadda, S.; Ramananda, Y.; Abu-El-Haija, M.; Palermo, J.J.; Appakalai, B.N.; Nathan, J.D. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat. Commun. 2019, 10, 1–12.
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 1–12.
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.-W.; Seol, Y.-J.; Zhang, Y.S.; Shin, S.-R.; Zhao, L.; Aleman, J. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 1–16.
- Ramme, A.P.; Koenig, L.; Hasenberg, T.; Schwenk, C.; Magauer, C.; Faust, D.; Lorenz, A.K.; Krebs, A.-C.; Drewell, C.; Schirrmann, K. Autologous induced pluripotent stem cell-derived four-organ-chip. Future Sci. OA 2019, 5, FSO413.
- Jang, K.-J.; Suh, K.-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010, 10, 36–42.
- Maschmeyer, I.; Lorenz, A.; Ramme, A.; Hasenberg, T.; Schimek, K.; Hübner, J.; Lauster, R.; Marx, U. A microfluidic four-organ-chip for interconnected long-termco-culture of human intestine, liver, skin and kidney equivalents. Toxicol. Lett. 2015, 2, S176.
- Junaid, A.; Mashaghi, A.; Hankemeier, T.; Vulto, P. An end-user perspective on Organ-on-a-Chip: Assays and usability aspects. Curr. Opin. Biomed. Eng. 2017, 1, 15–22.
- Miccoli, B.; Braeken, D.; Li, Y.-C.E. Brain-on-a-chip devices for drug screening and disease modeling applications. Curr. Pharm. Des. 2018, 24, 5419–5436.
- Jahromi, M.A.M.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Basri, S.M.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic brain-on-a-chip: Perspectives for mimicking neural system disorders. Mol. Neurobiol. 2019, 56, 8489–8512.
- Ndyabawe, K.; Kisaalita, W.S. Engineering Microsystems to recapitulate brain physiology on a chip. Drug Discov. Today 2019, 24, 1725–1730.
- Zhao, Y.; Demirci, U.; Chen, Y.; Chen, P. Multi scale brain research on a microfluidic chip. Lab Chip 2020, 20, 1531–1543.
- Wang, Y.; Wang, L.; Zhu, Y.; Qin, J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 2018, 18, 851–860.
- Jorfi, M.; D’Avanzo, C.; Tanzi, R.E.; Kim, D.Y.; Irimia, D. Human neurospheroid arrays for in vitro studies of Alzheimer’s disease. Sci. Rep. 2018, 8, 1–13.
- Kato-Negishi, M.; Tsuda, Y.; Onoe, H.; Takeuchi, S. A neurospheroid network-stamping method for neural transplantation to the brain. Biomaterials 2010, 31, 8939–8945.
- Lee, G.; Lim, J.; Park, J.; Lee, W.; Yoon, D.S.; Kim, S.H.; Kim, M.-K.; Lee, S.-H.; Kim, D.-H. Construction of neurospheroids via surface modified concave microwells. J. Ind. Eng. Chem. 2018, 62, 341–351.
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42.
- Jamieson, J.J.; Gerecht, S. Chipping away at blood-brain-barrier modeling. Cell Stem Cell 2019, 24, 831–832.
- Van der Helm, M.; vanderMeer, A.; Eijkel, J.; vandenBerg, A.; Segerink, L. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493.
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185.
- Bertini, G.; Bramanti, P.; Constantin, G.; Pellitteri, M.; Radu, B.M.; Radu, M.; Fabene, P.F. New players in the neurovascular unit: Insights from experimental and clinical epilepsy. Neurochem. Int. 2013, 63, 652–659.
- Muoio, V.; Persson, P.; Sendeski, M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798.
- Abbott, N.J. Astrocyte–endothelial interactions and blood–brain barrier permeability. J. Anat. 2002, 200, 523–534.
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuronmetabolic cooperation. Cell Metab. 2011, 14, 724–738.
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond black-and-white. J. Neurosci. 2018, 38, 14–25.
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18.
- BioRender Home Page. Available online: (accessed on 18 May 2021).
- DeFelice, F.G.; Munoz, D.P. Opportunities and challenges in developing relevant animal models for Alzheimer’s disease. Ageing Res. Rev. 2016, 26, 112–114.
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2015, 2, 258–270.
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflamm. 2016, 13, 1–17.
- Herland, A.; van derMeer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360.
- Wang, J.D.; Khafagy, E.-S.; Khanafer, K.; Takayama, S.; ElSayed, M.E. Organization of endothelialcells, pericytes, and astrocytes into a 3D microfluidic in vitro model of the blood–brain barrier. Mol. Pharm. 2016, 13, 895–906.
- Griep, L.M.; Wolbers, F.; de Wagenaar, B.; ter Braak, P.M.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Vermes, I.; van der Meer, A.D.; van den Berg, A. BBB on chip: Microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdevices 2013, 15, 145–150.
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; VanVught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G. A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 2018, 15, 1–12.
- Xu, H.; Li, Z.; Yu, Y.; Sizdahkhani, S.; Ho, W.S.; Yin, F.; Wang, L.; Zhu, G.; Zhang, M.; Jiang, L. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016, 6, 1–12.
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784–1792.
- Motallebnejad, P.; Thomas, A.; Swisher, S.L.; Azarin, S.M. An isogenic hiPSC-derived BBB-on-a-chip. Biomicrofluidics 2019, 13, 064119.
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidicblood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194.
- Jeong, S.; Kim, S.; Buonocore, J.; Park, J.; Welsh, C.J.; Li, J.; Han, A. A three-dimensional arrayed microfluidic blood–brain barrier model with integrated electrical sensor array. IEEE Trans. Biomed. Eng. 2017, 65, 431–439.
- Sellgren, K.L.; Hawkins, B.T.; Grego, S. An optically transparent membrane supports shear stress studies in a three-dimensional microfluidic neurovascular unit model. Biomicrofluidics 2015, 9, 061102.
- Falanga, A.P.; Pitingolo, G.; Celentano, M.; Cosentino, A.; Melone, P.; Vecchione, R.; Guarnieri, D.; Netti, P.A. Shuttle-mediated nanoparticle transport across an in vitro brain endothelium under flow conditions. Biotechnol. Bioeng. 2017, 114, 1087–1095.
- Moya, M.L.; Triplett, M.; Simon, M.; Alvarado, J.; Booth, R.; Osburn, J.; Soscia, D.; Qian, F.; Fischer, N.O.; Kulp, K. A reconfigurable in vitro model for studying the blood–brain barrier. Ann. Biomed. Eng. 2020, 48, 780–793.
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 1–12.
- Lee, S.; Chung, M.; Lee, S.R.; Jeon, N.L. 3D brain angiogenesis model to reconstitute functional human blood–brain barrier in vitro. Biotechnol. Bioeng. 2020, 117, 748–762.
- Nguyen, P.Q.H.; Duong, D.D.; Kwun, J.D.; Lee, N.Y. Hybrid elastomer–plastic microfluidic device as a convenient model for mimicking the blood–brain barrier in vitro. Biomed. Microdevices 2019, 21, 1–11.
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e1006.
- Kilic, O.; Pamies, D.; Lavell, E.; Schiapparelli, P.; Feng, Y.; Hartung, T.; Bal-Price, A.; Hogberg, H.T.; Quinones-Hinojosa, A.; Guerrero-Cazares, H. Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip 2016, 16, 4152–4162.
- Prabhakarpandian, B.; Shen, M.-C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A microfluidic blood brain barrier model. Lab Chip 2013, 13, 1093–1101.
- Deosarkar, S.P.; Prabhakarpandian, B.; Wang, B.; Sheffield, J.B.; Krynska, B.; Kiani, M.F. A novel dynamic neonatal blood-brain barrier on a chip. PLoS ONE 2015, 10, e0142725.
- Stoica, R.; Rusu, C.M.; Staicu, C.E.; Burlacu, A.E.; Radu, M.; Radu, B.M. Ca2+ homeostasis in brain microvascular endothelial cells. Int. Rev. Cell Mol. Biol. 2021; 362, part A, in press.
- Alcendor, D.J.; Block, F.E., III; Cliffel, D.E.; Daniels, J.S.; Ellacott, K.L.; Goodwin, C.R.; Hofmeister, L.H.; Li, D.; Markov, D.A.; May, J.C. Neurovascular unit on a chip: Implications for translational applications. Stem Cell Res. Ther. 2013, 4, 1–5.
- Huh, D.; Torisawa, Y.-S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered physiological biomimicry: Organs-on-chips. Lab Chip 2012, 12, 2156–2164.
- Gumbleton, M.; Audus, K.L. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J. Pharm. Sci. 2001, 90, 1681–1698.
More
Information
Subjects:
Neurosciences; Biophysics; Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
14 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




