Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Gamna | + 2881 word(s) | 2881 | 2021-07-09 04:57:08 | | | |
| 2 | Lily Guo | Meta information modification | 2881 | 2021-07-12 10:09:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gamna, F. Vitamin E. Encyclopedia. Available online: https://encyclopedia.pub/entry/11948 (accessed on 28 February 2026).
Gamna F. Vitamin E. Encyclopedia. Available at: https://encyclopedia.pub/entry/11948. Accessed February 28, 2026.
Gamna, Francesca. "Vitamin E" Encyclopedia, https://encyclopedia.pub/entry/11948 (accessed February 28, 2026).
Gamna, F. (2021, July 12). Vitamin E. In Encyclopedia. https://encyclopedia.pub/entry/11948
Gamna, Francesca. "Vitamin E." Encyclopedia. Web. 12 July, 2021.
Copy Citation
Vitamin E is a common compound used for tocopherols and tocotrienols (α, β, γ, δ); it is the component of many natural products of both plant and animal origin.
vitamin E
biomaterials
biomedical applications
1. Introduction
1.1. Structure of Vitamin E
Vitamin E was first discovered and described by Evans and Bishop in 1922; it includes eight natural forms (α, β, γ, δ; tocopherols and tocotrienols), and it can be found in various products bearing fats of both vegetable and animal origin, such as in olive or almond oil, hazelnuts, and egg yolk, and in the liver. Basically, tocopherols and tocotrienols have the same chemical structure, characterized by a 16-carbon lateral chain attached to position 2 of a benzopyran ring. The two isoforms differ substantially from the saturation of the long radical chain: tocopherols have a fully saturated chain, while tocotrienols have an unsaturated chain. As Figure 1 explains, the two homologs were named according to the position and number of the methyl group bound to the phenolic ring [1][2][3].
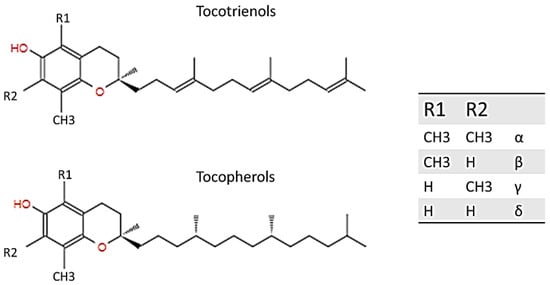
Figure 1. Structure of tocotrienol and tocopherol.
Among all the isoforms of vitamin E, α-tocopherol is the most abundant in the blood, because it is the only one that is absorbed within the body, while the other isoforms are excreted through the intestine. The liver, which takes up nutrients after they are absorbed by the small intestine, absorbs vitamin E thanks to the plasmatic lipoproteins that function as carriers. Among all the various forms of the vitamin E, alpha-tocopherol is re-secreted through the liver protein of α-tocopherol transfer (α-TTP) and distributed to circulating lipoproteins (LDL, IDL, VLDL). The other forms are metabolized and then expelled through the intestine (Figure 2) [4].
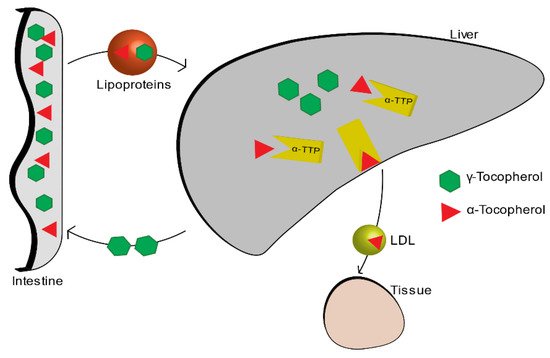
Figure 2. Vitamin E metabolism.
1.2. Biological Role of Vitamin E
Alpha-tocopherol is a phenolic antioxidant. The scavenging mechanism involves the donation of hydrogen from the hydroxyl group (-OH) of the phenolic ring to free radicals (ROS). In this way, the free radicals become unreactive and unable to do any more damage. After this reaction, also the phenolic compound itself becomes relatively unreactive with a higher stability. Its stability is guaranteed by the now unpaired electron which is on the oxygen atom and which is delocalized in the structure of the aromatic ring. α-tocopherol is located within the phospholipid membrane of the cell, and it occurs with the radical chain embedded in the hydrophobic core of the double layer [5]. Its concentration, compared to the lipids present in the membrane, is very low, but in spite of this, it plays an important role in preserving the integrity of the membrane by preventing lipid peroxidation which causes damage of cellular membranes, lipoproteins, and other molecules that contain lipids, in conditions of oxidative stress [1][6][7].
Oxidative stress is a pathological condition caused by the imbalance between the generation and elimination of chemical oxidant species (ROS), and it is involved in several neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease that are implicated in free radical processes and oxidative damage [8]. That said, it is easy to think of vitamin E that, thanks to its important qualities as an antioxidant, may have an important role in the integrity of the brain. To confirm this, a high level of α-TTP was found in the brain [9].
Vitamin E is an important anti-inflammatory molecule since it acts on many different factors that affect, directly or indirectly, the immune system. Vitamin E is able to modulate inflammation through different ways: it has effect on proinflammatory enzymes such as COX, responsible for prostaglandins (PG)E2 production [10][11]; (PG)E2 is a proinflammatory mediator that has been associated with several senility-related diseases such as cancer, arthritis, and cardiovascular diseases [4][7][12]). It modulates the proliferation and activation of certain cells of the immune system such as T cells, lymphocytes, and NK cells [13]. Finally, it acts on the secretion of proinflammatory cytokines such as IL-6 and TNF-α. Thanks to these factors, vitamin E plays an important role in helping to prevent chronic inflammation [11][13]. Chronic inflammation is strictly linked to oxidative stress [14] and, together with it, it is the main cause of age-related disease and cancer [11].
Vitamin E has also an important role in the inhibition of platelet aggregation, inhibiting various enzymes such as PKC, which is a key signal transduction pathway in several cell types [1][15]. α-tocopherol, thanks to its free-radical scavenging and anti-inflammatory properties, has a benefit also in dermatology: it protects the skin from UV radiation, and accelerates the wound healing process after an injury such as ulcers or burns [16]. Inflammation could be associated also to a large number of different phenomena related to bone health: it is thought that thanks to its anti-inflammatory action and regulation of cytokine secretion, vitamin E can influence bone remodelling, being able to protect the bone against osteoclastic activity, increasing osteoblasts differentiation, and protecting cartilage health [17][18][19]. In addition, recent studies have shown that vitamin E also has biomechanical effects, being able to increase specific characteristics such as load and yield [20]. Together with all these considerations, vitamin E, due to its antioxidant function, role in anti-inflammatory processes, inhibition of platelet aggregation, and immune system-enhancing activity [1][9], brings a wide range of benefits, from anti-cancer effects [21] to the prevention of disease progression, and in improving quality of life in the elderly [22]. The figure below (Figure 3) shows a schematic representation of the biological role of vitamin E.
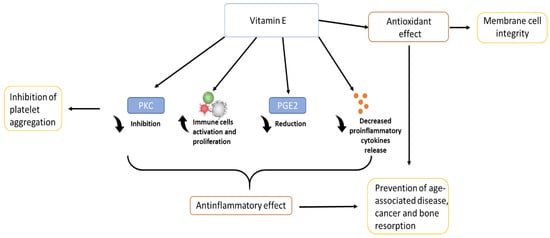
Figure 3. Biological role of vitamin E.
More controversial and still under study, is the anti-bacterial role of vitamin E. In the literature, it is possible to find vitamin E used as a natural compound to treat infections caused by specific Gram-positive or negative bacteria [23][24] or as an antibiotic adjuvant used in combination with antibiotics for the treatment of infections [25][26]. Moreover, the addition of vitamin E to materials may have the ability to reduce biofilm formation on the surface. As the literature suggests, it is possible to believe that tocopherol is able to reduce the biofilm formation capacity of a big range of strains (S. aureus and S. epidermidis, etc.), regardless of the classification of the bacterium (Gram-negative or -positive) [27]. This, however, comes into opposition with other studies that show instead that vitamin E, in composition with other materials, did not reduce biofilm formation [28].
Once the biological role of vitamin E is understood, it is certainly interesting to analyse its application and detection methods, when it is combined to a biomaterial. Over the past 20 years, there has been a strong increase in interest in scientific research on α-tocopherol combined with various biomaterials (Figure 4). This review selected about 100 reviews best suited to the topic discussed.
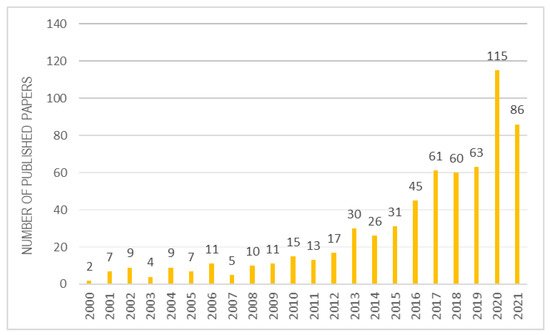
Figure 4. Studies inherent to α-tocopherol combined with biomaterials over the years.
2. Methods to Detect and Quantify Vitamin E within Materials
Vitamin E can be easily detected in different liquid media, such as in oils, serum, human milk, foods, etc., by different methods including HPLC, FTIR, RAMAN, UV-VIS, and spectrophotometric methods. Recently, some protocols were also developed for the analysis of vitamin E incorporated into cosmetics and food packaging and contained in food [29][30][31][32][33][34].
The first method usually used to allow a simple and rapid quantitative determination of α-tocopherol is High-Performance Liquid Chromatography (HPLC). In fact, HPLC is one of the most powerful tools for the determination of fat-soluble vitamins and has been widely used for their separation and detection; different detectors can be used for vitamins such as UV-VIS, fluorescence, and mass spectrometry. In the case of vitamin E, typically the HPLC column is connected to an UV absorbance detector as the compound absorbs the ultraviolet light, particularly around 290 nm. This method is, in fact, used not only to analyse quantitatively the content of alpha-tocopherol in food or beverages, but also in cosmetics and in biological samples including human plasma and human milk [31][34][35][36][37]. Another way to detect and quantify α-tocopherol is the Fourier Transform Infrared Spectroscopy (FTIR). Sandra et al. developed a quick procedure for the quantitative analysis of α-tocopherol in vegetable oils as an alternative to HPLC methods, using FTIR-ATR methodology. By analysing 13 vegetable oils, with a known content of vitamin E, a research team created a calibration curve which was then used to measure the alpha-tocopherol content of the vegetable oil concerned quantitatively [38].
For qualitative measurements, FTIR was also used for detecting the functional groups of a hydrophobic film of vitamin E deposited on a copper substrate [39]. Thanks to its clear absorbance peak at 290 nm, visible ultraviolet spectroscopy (UV-Vis) proved to be able to detect the presence of vitamin E even at low concentrations [40][41].
Along with FTIR, RAMAN is a potential alternative method to have qualitative detection of the molecule. It is used to detect vitamin E in oil water emulsions and in biological samples [42][43]. Surface-enhanced Raman spectroscopy (SERS) technology is of a high level of interest: it exploits the amplification of Raman diffusion by molecules adsorbed on a metal or on metallic nanoparticles [44][45]. Typically, most SERS techniques use metal aqueous colloids as a substrate, which require that compounds to be analysed must be water soluble. For water insoluble analytes, such as vitamin E, the matter is more complicated. Given the disadvantage of using colloidal Ag nanoparticles to measure SERS of the analyte directly, Tiantian Cai et al. have successfully tried other methods to analyse vitamin E: after dissolving the compound in chloroform, the solution obtained is dripped onto the surface of a metal substrate with surface Raman activity. Another method could be to immerse the metal substrate in the sample solution containing vitamin E directly, to extract it after a certain time, and to measure it at RAMAN after the solvent has evaporated [46].
Thanks to its antioxidant properties, vitamin E can also be analysed and quantified through all those methods that exploit chemical reactions, typically redox, to develop coloured compounds that are then measured spectrophotometrically. In general, spectrophotometric methods for vitamin E determination use oxidation of the aromatic ring of α-tocopherol, creating tocopherylquinone, by oxidizing agents that ultimately yield products with spectrophotometric staining. Among these methods, there is definitely the DPPH method, which uses a free radical of purple colour, which discolours when it reacts with vitamin E. Valeria M. et al. have used the DPPH method to compare the antioxidant power of drugs containing alpha-tocopherol. The problem of the DPPH method is its low reproducibility due to the low stability of the radical [47].
Another such methodology is the Folin–Ciocâlteu (FC) reagent in an aqueous solution. In this case, however, given the insolubility of vitamin E in water, this methodology is not the optimal one. However, modifications have been made to the FC method to enable the measurement of lipophilic and hydrophilic antioxidants concentrations simultaneously [48].
Albeit less used and dated, there are many other methods using different oxidizing reagents such as Fe(III)-bathophenanthroline, Cu(II)-neocuproine, or silver nitrate, but they require a rigid control of the conditions for precise results [49]. Another method is the Emmerie and Engel colour reaction with ferric chloride: it is a precise and easy-to-perform reaction, and therefore the approach of choice for a routine clinical laboratory [50]. Based on this work, more recently, Jameel G. Jargar et al. took advantage of different reagents such as 2,2′-bipyridyl, ferric chloride, and xylene to perform the colour reaction [51].
Finally, although a very old and no longer used method, nitric acid combined with ethanol was used to oxidise α-tocopherol, forming the coloured red o-quinone which can be detected spectrophotometrically (Figure 5) [52].
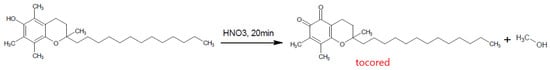
Figure 5. Formation of Tocored with Nitric Acid.
Thanks to the vitamin E detection methods employed in various applications involving cosmetics, food packaging, and so on, it is possible to apply the above methods to the biomedical field for the detection of vitamin E when combined with different biomaterials.
Certainly, it is easy to find detection methods in the literature when vitamin E is combined with UHWMPE, as it is the most widely applied biomaterial coupled with vitamin E today.
For quantification methods, with HPLC analysis, it is convenient to quantify the vitamin E content within UHMWPE using a calibration curve produced from the areas of the HPLC peaks [53]. Instead, Hufen Julia et al. developed an accurate method to detect α-tocopherol content in UHWMPE using HPLC analysis to separate it and determine its concentration by UV-Vis spectroscopy with a corresponding calibration curve [54]. However, it is also possible to use only UV-VIS in absorbance mode combined with FTIR to quantify vitamin E within UHMWPE [55]. Vitamin E blended with polyethylene induces yellowing of the sample; Martínez-Morlanes et al. exploited this characteristic using the colorimetric technique and reflectance spectroscopy to detect vitamin E embedded in polyethylene samples quantitatively [56]. These types of methods, especially HPLC, are also used in drug delivery to calculate the drug encapsulation efficiency, resulting in the quantification of the vitamin E encapsulated within polymeric nanoparticles [57][58][59]. With the same object, in tissue engineering, HPLC is used to quantify vitamin E content inside the matrices or scaffolds [60][61].
For qualitative methods, since vitamin E is an extremely hydrophobic molecule, another important way to detect the presence of tocopherol on different substrates is definitely the measurement of the contact angle, the quickest test to evaluate a surface modification [62]. Filippo Renò et al. used the contact angle measurement on PLA blended with Vitamin E, and they discovered that the blend enriched with vitamin E was more easily wetted [63][64].
To get a more in-depth understanding of the chemical bonds between the substrate and the deposited molecule, the XPS technique is useful, as in the case of Elena Stoleru et al. who used XPS to have information about the stability of the chitosan/vitamin E coating deposited on a polyethylene substrate [62].
Once the characteristic peaks of vitamin E are known, the FTIR analysis is helpful, not only to detect vitamin E [65], but also to analyse the eventual shifts in wavenumber of the peaks that denote an interaction between tocopherol and the combined biomaterials. Ahmad Salawi et al. used the FTIR technique to analyse the interaction between a new copolymer called Soluplus and α-tocopherol for a wound-healing application [66], and Joana T. Martins et al. studied the physiochemical effect of the incorporation of α-tocopherol in chitosan-based films through a different analysis including FTIR [67].
In the biomedical field, the DPPH test is used to analyse the radical scavenging ability of vitamin E combined with biomaterials, as Elena Stoleru et al. did on a film electrosprayed with a chitosan/vitamin E formulation [62]. DPPH was used also by Zhou Nier et al. to test the antioxidant activity of Au nanoparticles functionalized with Trolox (hydrophilic analogue of alpha-tocopherol) [68][69]. The table (Table 1) reports the characterization methods used to detect vitamin E when combined with different biomaterials.
Table 1. Method of Vitamin E detection when it is combined with biomaterials.
| Technique | Combined Material | Molecule Detected | Method | Information | Ref. |
|---|---|---|---|---|---|
| HPLC | UHWMPE | α-tocopherol | HPLC connected to UV/Vis diode array detector at 297 nm, construction of calibration curve of HPLC peak area. | Quantitative | [53] |
| UHWMPE | α-tocopherol | HPLC connected to UV/Vis diode array detector, construction of calibration curve of absorbance peak area at 290 nm | Quantitative | [54] | |
| Collagen mesh | α-tocopherol | HPLC connected to a fluorescence detector, detection at excitation wavelength of 290 nm and emission wavelength of 330 nm | Quantitative | [61] | |
| Alginate and hyaluronate film | α-tocopherol acetate | HPLC connected to UV/Vis diode array detector, construction of calibration curve of absorbance peak area at 285 nm | Quantitative | [60] | |
| Hyaluronic-acid-based β-cyclodextrin copolymer | α-tocopherol | HPLC connected to UV/Vis diode array detector | Quantitative | [58] | |
| PNIPAM-b-PCL-b-PNIPAM copolymer | α-tocopherol | HPLC equipped with a differential refraction index detector | Quantitative | [59] | |
| UV-VIS | UHWMPE | α-tocopherol | Construction of calibration curve of absorbance peak area at 290 nm | Quantitative | [55] |
| UHWMPE | α-tocopherol | Analysis of reflectance spectra which presents a minimum around 290 nm and a decrease of reflectance at 400–500 nm. | Detection | [56] | |
| Hyaluronic acid | α-tocopherol succinate | Construction of calibration curve of absorbance peak area at 285 nm | Quantitative | [70] | |
| PLA+PCL | α-tocopherol acetate | Construction of calibration curve of absorbance peak area at 284 nm | Quantitative | [65] | |
| Colorimetric Assay | UHWMPE | α-tocopherol | The yellowing of the sample was analysed through three parameters (a,b,L) of CIELAB colour space, and a calibration curve of colour distances was constructed. | Quantitative | [56] |
| FTIR-ATR | UHWMPE | α-tocopherol | Analysis of peaks. For quantitative analysis, calibration curve of these peaks is needed. |
Analysis of Vitamin E transformation products in polymer samples prior to extraction and quantitative. | [53] |
| Collagen | α-tocopherol | Analysis of main peaks | Characterization of film | [71] | |
| FTIR-ATR | Magnetite | α -tocopheryl succinate | Analysis of main peaks | Characterization of chemical modification of nanoparticles | [72] |
| Chitosan | α-tocopherol | Analysis of peaks | Physical bonds and chemical interactions are reflected by changes in characteristic spectral peaks. | [67] | |
| Chitosan | α-tocopherol | Analysis of peaks | Characterization of nanoparticles | [73] | |
| PCL/PLA | α-tocopherol acetate | Analysis of peaks | Characterization of membranes | [65] | |
| Soluplus | α-tocopherol | Analysis of peaks | Analysis of bonding between Soluplus/vitamin E | [66] | |
| Polyethylene | α-tocopherol | Analysis of peaks from 600–4000 cm −1 | Analysis of interaction between vitamin E and chitosan | [62] | |
| XPS | Polyethylene | α-tocopherol | All binding energies were referenced to the C1s peak at 285 eV. | Analysis of covalent bonding | [62] |
| DPPH | Polyethylene | α-tocopherol | The scavenging activity was estimated RSA (%) = (1 − (A sample/Acontrol)) × 100, measuring the adsorption at 515 nm after 30 min in dark condition. |
Radical scavenging activity evaluation | [62] |
| Chitosan | α-tocopherol | The scavenging activity was estimated RSA (%) = (1 − (A sample/Acontrol)) × 100, measuring the adsorption at 517 nm after 30 min in dark condition. |
Radical scavenging activity evaluation | [67] | |
| Collagen/chitosan | α-tocopherol | DPPH were measured by the adsorption at 517 nm after 30 min in dark condition. DPPH loss which is a concentration of DPPH radicals reacted with antioxidants. | Antioxidant activity | [61] | |
| Contact Angle | Polyethylene | α-tocopherol | Contact angle titrations were performed by measuring sets of contact angles at each pH value. | Analysis of hydrophobic behaviour as pH increases | [51] |
| PLA | α-tocopherol | Static contact angle | Analysis of material wettability change | [63][64] |
References
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of Vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, 157–165.
- Mutalip, S.S.M.; Ab-Rahim, S.; Rajikin, M.H. Vitamin E as an antioxidant in female reproductive health. Antioxidants 2018, 7, 22.
- Colombo, M.L. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules 2010, 15, 2103–2113.
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90.
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336.
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860.
- Wang, X.; Quinn, P.J. The location and function of vitamin E in membranes (Review). Mol. Membr. Biol. 2000, 17, 143–156.
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975.
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The effectiveness of vitamin E treatment in alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 879.
- Schubert, M.; Kluge, S.; Schmölz, L.; Wallert, M.; Galli, F.; Birringer, M.; Lorkowski, S. Long-chain metabolites of vitamin E: Metabolic activation as a general concept for lipid-soluble vitamins? Antioxidants 2018, 7, 10.
- Reitera, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of α- and γ-tocopherol. Bone 2007, 28, 668–691.
- Wu, D.; Hayek, M.G.; Meydani, S.N. Symposium: Molecular mechanisms of protective effects of vitamin E in atherosclerosis: Vitamin E and macrophage cyclooxygenase regulation in the aged. J. Nutr. 2001, 131, 382–388.
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614.
- Hardbower, D.M.; de Sablet, T.; Chaturvedi, R.; Wilson, K.T. Chronic inflammation and oxidative stress: The smoking gun for helicobacter pylori-induced gastric cancer? Gut Microbes 2013, 4, 475–481.
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15.
- Keen, M.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311.
- Nazrun, A.S.; Norazlina, M.; Norliza, M.; Nirwana, S.I. The anti-inflammatory role of vitamin e in prevention of osteoporosis. Adv. Pharmacol. Sci. 2012, 2012, 142702.
- Wong, S.K.; Mohamad, N.V.; Ibrahim, N.; Chin, K.Y.; Shuid, A.N.; Ima-Nirwana, S. The molecular mechanism of Vitamin E as a bone-protecting agent: A review on current evidence. Int. J. Mol. Sci. 2019, 20, 1453.
- Rossato, M.F.; Hoffmeister, C.; Tonello, R.; Ferreira, A.P.d.; Ferreira, J. Anti-inflammatory Effects of Vitamin E on Adjuvant-Induced Arthritis in Rats. Inflammation 2015, 38, 606–615.
- Feresin, R.G.; Johnson, S.A.; Elam, M.L.; Kim, J.S.; Khalil, D.A.; Lucas, E.A.; Smith, B.J.; Payton, M.E.; Akhter, M.P.; Arjmandi, B.H. Effects of Vitamin e on Bone Biomechanical and Histomorphometric Parameters in Ovariectomized Rats. J. Osteoporos. 2013, 2013, 825985.
- Constantinou, C.; Papas, A.; Constantinou, A.I. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer 2008, 123, 739–752.
- Capuron, L.; Moranis, A.; Combe, N.; Cousson-Gélie, F.; Fuchs, D.; De Smedt-Peyrusse, V.; Barberger-Gateau, P.; Layé, S. Vitamin E status and quality of life in the elderly: Influence of inflammatory processes. Br. J. Nutr. 2009, 102, 1390–1394.
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Pereira, R.L.; Costa, M.; Braga, M.F.B.M.; Limaverde, P.W.; Andrade, J.C.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016, 15, 315–322.
- Abd, D.; Kader, A.; Aziz, F.M. Antibacterial Effects of Vitamin E: In Vitro Study. J. Biotechnol. Res. Cent. 2013, 7, 17–23.
- Naguib, M.M.; Valvano, M.A. Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding. mSphere 2018, 3, e00564-18.
- Andrade, J.C.; Morais-Braga, M.F.; Guedes, G.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.; Coutinho, H.D. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed. Pharmacother. 2014, 68, 1065–1069.
- Vergalito, F.; Pietrangelo, L.; Petronio Petronio, G.; Colitto, F.; Alfio Cutuli, M.; Magnifico, I.; Venditti, N.; Guerra, G.; Di Marco, R. Vitamin E for prevention of biofilm-caused Healthcare-associated infections. Open Med. 2020, 15, 14–21.
- Williams, D.L.; Vinciguerra, J.; Lerdahl, J.M.; Bloebaum, R.D. Does Vitamin E-blended UHMWPE Prevent Biofilm Formation? Clin. Orthop. Relat. Res. 2015, 473, 928–935.
- Lee, C.H.; An, D.S.; Lee, S.C.; Park, H.J.; Lee, D.S. A coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and α-tocopherol. J. Food Eng. 2004, 62, 323–329.
- Sawicki, T.; Bączek, N.; Starowicz, M. Characterisation of the total phenolic, vitamins C and E content and antioxidant properties of the beebread and honey from the same batch. Czech J. Food Sci. 2020, 38, 158–163.
- Lampen, P.; Pittermann, W.; Heise, H.M.; Schmitt, M.; Jungmann, H.; Kietzmann, M. Penetration studies of vitamin E acetate applied from cosmetic formulations to the stratum corneum of an in vitro model using quantification by tape stripping, UV spectroscopy, and HPLC. J. Cosmet. Sci. 2003, 54, 119–131.
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of phenolic compounds, ascorbic acid and vitamin E to antioxidant activity of currant (Ribes L.) and gooseberry (Ribes uva-crispa L.) fruits. Food Chem. 2019, 284, 323–333.
- Marcos, B.; Sárraga, C.; Castellari, M.; Kappen, F.; Schennink, G.; Arnau, J. Development of biodegradable films with antioxidant properties based on polyesters containing α-tocopherol and olive leaf extract for food packaging applications. Food Packag. Shelf Life 2014, 1, 140–150.
- Korchazhkina, O.; Jones, E.; Czauderna, M.; Spencer, S.A.; Kowalczyk, J. HPLC with UV detection for measurement of vitamin E in human milk. Acta Chromatogr. 2006, 16, 48–57.
- Romeu-Nadal, M.; Morera-Pons, S.; Castellote, A.I.; López-Sabater, M.C. Determination of γ- and α-tocopherols in human milk by a direct high-performance liquid chromatographic method with UV-vis detection and comparison with evaporative light scattering detection. J. Chromatogr. A 2006, 1114, 132–137.
- Delgado Zamarreño, M.M.; Sanchez Ferez, A.; Sanchez Rodriguez, M.; Gomez Ferez, M.C.; Hernandez Mendez, J. Determination of fat-soluble vitamins in yogurt by HPLC with electrochemical detection. Talanta 1996, 43, 1555–1563.
- Campanero, M.A.; Calahorra, B.; Garcia-Quetglas, E.; Honorato, J.; Carballal, J.J. Determination of cisapride in human plasma by high-performance liquid chromatography. Chromatographia 1998, 47, 537–541.
- Silva, S.D.; Rosa, N.F.; Ferreira, A.E.; Boas, L.V.; Bronze, M.R. Rapid determination of α-tocopherol in vegetable oils by fourier transform infrared spectroscopy. Food Anal. Methods 2009, 2, 120–127.
- Fuchs-Godec, R.; Zerjav, G. Corrosion resistance of high-level-hydrophobic layers in combination with Vitamin E-(α-tocopherol) as green inhibitor. Corros. Sci. 2015, 97, 7–16.
- Jović, O.; Habinovec, I.; Galić, N.; Andrašec, M. Maceration of extra virgin olive oil with common aromatic plants using ultrasound-assisted extraction: An uv-vis spectroscopic investigation. J. Spectrosc. 2018, 2018.
- Li, X.; Chen, D.; Wang, G.; Lu, Y. Investigation on the interaction between bovine serum albumin and 2,2-diphenyl-1-picrylhydrazyl. J. Lumin. 2014, 156, 255–261.
- Wang, K.; Sun, D.W.; Wei, Q.; Pu, H. Quantification and visualization of α-tocopherol in oil-in-water emulsion based delivery systems by Raman microspectroscopy. LWT 2018, 96, 66–74.
- Beattie, J.R.; Maguire, C.; Gilchrist, S.; Barrett, L.J.; Cross, C.E.; Possmayer, F.; Ennis, M.; Elborn, J.S.; Curry, W.J.; McGarvey, J.J.; et al. The use of Raman microscopy to determine and localize vitamin E in biological samples. FASEB J. 2007, 21, 766–776.
- Lv, M.Y.; Zhang, X.; Ren, H.R.; Liu, L.; Zhao, Y.M.; Wang, Z.; Wu, Z.L.; Liu, L.M.; Xu, H.J. A rapid method to authenticate vegetable oils through surface-enhanced Raman scattering. Sci. Rep. 2016, 6, 23405.
- Tu, Q.; Lin, Z.; Liu, J.; Dai, H.; Yang, T.; Wang, J.; Decker, E.; McClements, D.J.; He, L. Multi-phase detection of antioxidants using surface-enhanced Raman spectroscopy with a gold nanoparticle-coated fiber. Talanta 2020, 206, 120197.
- Cai, T.; Gu, H.; Yuan, X.; Liu, F. Normal Raman and SERS spectroscopy of the vitamin E. J. Phys. Conf. Ser. 2011, 277.
- Di Mambro, V.M.; Azzolini, A.E.C.S.; Valim, Y.M.L.; Fonseca, M.J.V. Comparison of antioxidant activities of tocopherols alone and in pharmaceutical formulations. Int. J. Pharm. 2003, 262, 93–99.
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791.
- Tütem, E.; Apak, R.; Günaydi, E.; Sözgen, K. Spectrophotometric determination of vitamin E (α-tocopherol) using copper(II)-neocuproine reagent. Talanta 1997, 44, 249–255.
- Martinek, R.G. Method for the Determination of Vitamin E (Total Tocopherols) in Serum. Clin. Chem. 1964, 10, 1078–1086.
- Jargar, J.G.; Hattiwale, S.H.; Das, S.; Dhundasi, S.A.; Das, K.K. A modified simple method for determination of serum α-tocopherol (Vitamin E). J. Basic Clin. Physiol. Pharmacol. 2012, 23, 45–48.
- Golumbic, C.; Mattill, H.A. the Oxidation of Vitamin E. J. Biol. Chem. 1940, 134, 535–541.
- Doudin, K.; Al-Malaika, S. Vitamin E-stabilised UHMWPE for orthopaedic implants: Quantitative determination of vitamin E and characterisation of its transformation products. Polym. Degrad. Stab. 2016, 125, 59–75.
- Gmbh, T.; Llc, T.; Analytik, I.K. HPLC Method to Determine the Alpha-Tocopherol Content in UHMWPE. In Proceedings of the 55th Annual Meeting of the Orthopaedic Research Society, Las Vegas, NV, USA, 22–25 February 2009. Poster No. 2316.
- Rowell, S.; Oral, E.; Ok, M. Detection of Vitamin E in Irradiated UHMWPE by UV-Visible Spectroscopy. Chem. Pharm. Bull. 2004, 25, 2434–2439.
- Martínez-Morlanes, M.J.; Terriza, A.; Yubero, F.; Puértolas, J.A. Characterization of highly crosslinked polyethylenes by colorimetry. Polym. Test. 2012, 31, 841–847.
- Byun, Y.; Hwang, J.B.; Bang, S.H.; Darby, D.; Cooksey, K.; Dawson, P.L.; Park, H.J.; Whiteside, S. Formulation and characterization of α-tocopherol loaded poly e-caprolactone (PCL) nanoparticles. LWT Food Sci. Technol. 2011, 44, 24–28.
- Singh, P.; Wu, L.; Ren, X.; Zhang, W.; Tang, Y.; Chen, Y.; Carrier, A.; Zhang, X.; Zhang, J. Hyaluronic-acid-based β-cyclodextrin grafted copolymers as biocompatible supramolecular hosts to enhance the water solubility of tocopherol. Int. J. Pharm. 2020, 586, 119542.
- Quintero, C.; Vera, R.; Perez, L.D. α-Tocopherol loaded thermosensitive polymer nanoparticles: Preparation, in vitro release and antioxidant properties. Polimeros 2016, 26, 304–312.
- Pereira, G.G.; Guterres, S.S.; Balducci, A.G.; Colombo, P.; Sonvico, F. Polymeric films loaded with vitamin e and aloe vera for topical application in the treatment of burn wounds. BioMed Res. Int. 2014, 2014, 641590.
- Gim, S.Y.; Jung, J.; Kwon, Y.J.; Kim, M.J.; Kim, G.H.; Lee, J.H. Effects of chitosan and collagen containing α-tocopherol on the oxidative stability in bulk oil and oil-in-water emulsion. Food Sci. Biotechnol. 2018, 27, 947–956.
- Stoleru, E.; Munteanu, S.B.; Dumitriu, R.P.; Coroaba, A.; Drobotă, M.; Zemljic, L.F.; Pricope, G.M.; Vasile, C. Polyethylene materials with multifunctional surface properties by electrospraying chitosan/vitamin E formulation destined to biomedical and food packaging applications. Iran. Polym. J. 2016, 25, 295–307.
- Aina, V.; Gatti, S.; Cannas, M.; Reno, F. Effect of vitamin E addition to poly (D,L) -lactic acid on surface properties and osteoblast behaviour. Biomaterials 2005, 26, 5594–5599.
- Paul, G.; Rizzi, M.; Gatti, G.; Marchese, L.; Ren, F. Poly (D,L) Lactic Acid Blending with Vitamin E Increases Polymer Hemocompatibility: An Hydrophilic Effect. J. Appl. Polym. Sci. 2013, 129, 1527–1533.
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J.; et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447.
- Salawi, A.; Nazzal, S. The rheological and textural characterization of Soluplus®/Vitamin E composites. Int. J. Pharm. 2018, 546, 255–262.
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227.
- Nie, Z.; Liu, K.J.; Zhong, C.J.; Wang, L.F.; Yang, Y.; Tian, Q.; Liu, Y. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic. Biol. Med. 2007, 43, 1243–1254.
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant activity of metal nanoparticles coated with tocopherol-like residues—The importance of studies in homo-and heterogeneous systems. Antioxidants 2020, 9, 5.
- Sun, Q.; Bi, H.; Wang, Z.; Li, C.; Wang, X.; Xu, J.; Zhu, H.; Zhao, R.; He, F.; Gai, S.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473.
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Isacchi, B.; Bilia, A.R.; Picci, N. Collagen α-tocopherulate for topical applications: Preparation, characterization, and antioxidant activity evaluation. Macromol. Res. 2012, 20, 939–943.
- Angulo-Molina, A.; Méndez-Rojas, M.Á.; Palacios-Hernández, T.; Contreras-López, O.E.; Hirata-Flores, G.A.; Flores-Alonso, J.C.; Merino-Contreras, S.; Valenzuela, O.; Hernández, J.; Reyes-Leyva, J. Magnetite nanoparticles functionalized with α-tocopheryl succinate (α-TOS) promote selective cervical cancer cell death. J. Nanoparticle Res. 2014, 16.
- Faramarzi, M.A.; Naghibzadeh, M.; Amani, A.; Amini, M.; Esmaeilzadeh, E.; Mottaghi-Dastjerdi, N. An insight into the interactions between-tocopherol and chitosan in ultrasound-prepared nanoparticles. J. Nanomater. 2010, 2010, 818717.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.6K
Revisions:
2 times
(View History)
Update Date:
12 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





