
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pedro Póvoa | + 3232 word(s) | 3232 | 2021-07-10 12:57:24 | | | |
| 2 | Lindsay Dong | Meta information modification | 3232 | 2021-07-12 03:26:37 | | |
Video Upload Options
In recent years, the knowledge of pharmacokinetics and pharmacodynamics, drug dosing, therapeutic drug monitoring, and antimicrobial resistance in the critically ill patients has greatly improved, fostering strategies to optimize therapeutic efficacy and to reduce toxicity and adverse events. Nonetheless, delivering adequate and appropriate antimicrobial therapy is still a challenge, since pathogen resistance continues to rise, and new therapeutic agents remain scarce.
1. Introduction
2. Pharmacokinetic and Pharmacodynamic Characteristics of Antimicrobials
The therapeutic window of a drug is defined according to previously studied dose–response relationships which will also determine the limits of safe concentration and dosage. The dose and duration of dosing intervals of AM are determined according to their pharmacokinetic/pharmacodynamic (PK/PD) properties [6]. However, in critical illness, multiple underlying derangements provoke pathophysiological alterations that change the PK/PD of drugs and therefore provoke dynamic changes in drug concentration.
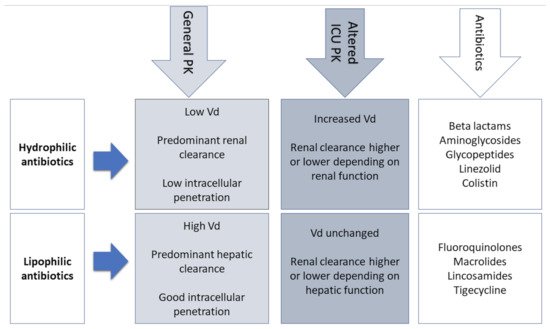
3. PK Changes in Critically Ill Patients
| Antibiotics that Stay in Extracellular Fluid (Vd < 0.3 L/kg) |
Drugs that Distribute into Total Body Water (Vd 0.7–1 L/kg) |
Drug with High Distribution to Tissues (Vd > 1 L/kg) |
|---|---|---|
|
|
|
Another important factor that can influence the Vd of AM is the modification in protein binding. Since albumin is the main plasma-binding protein for many AM (e.g., cefazolin, ceftriaxone, ertapenem, and daptomycin), its decreased concentration in septic patients has a direct impact on the PK of antibiotics [13]. With low plasma albumin, there is an increase of the unbound antibiotic, increasing its Vd and clearance, and leading to lower and probably suboptimal AM concentrations toward the end of dosing intervals. For these reasons, therapeutic drug monitoring (TDM) should include an adjustment for low albumin levels or a direct measurement of free drug levels [14].
4. Antibiotics in the ICU
4.1. Β-Lactams
The β-lactams’ broad spectrum of AM activity and low toxicity profiles unsurprisingly render them first-line options in serious infections, namely Gram-negative bacilli (GNB) infections, and the most commonly prescribed AM in critical care [15][16].
Β-lactams are generally hydrophilic, with low Vd, moderate to low protein binding, and essentially renal excretion [17]. In vivo animal studies have clearly shown that β-lactams are characterized by a slow continuous kill, in other words, time-dependent bactericidal activity [18][19]. Consequently %fT > MIC is the optimal PK/PD parameter for β-lactams, with the recommended interval of 40–70% [17][20], varying according to the AM and underlying pathogens [21]. This time-dependent effect is independent of peak values and little post-antibiotic effect exists, except for carbapenems [22]. Since β-lactams have short or no post-antibiotic effect, when AM concentration falls below the MIC at the infection site, residual pathogens can rapidly regrow [23]. Furthermore, frequent Vd and Cl alterations accentuate the risk of suboptimal drug concentrations in the face of critical illness [17]. For example, with hypoalbuminemia, highly protein-bound β-lactams such as ceftriaxone, ertapenem, flucloxacillin, and oxacillin will present increased free fractions [24].
4.2. Aminoglycosides
4.3. Glycopeptides
Vancomycin
4.4. Colistin
4.5. Fluoroquinolones
5. Strategies to Optimize Dosing
| Antimicrobial Class | Monitoring/ Sampling |
PK/PD Target | Toxicity Threshold |
|---|---|---|---|
| Therapeutic Drug Monitoring Recommended | |||
Beta-lactams
|
Cmin/One sample 1 Css (continuous infusion)/One sample 2 |
100% fT> MIC Css > MIC 50–100% fT > MIC 45–100% fT > MIC 50–100% fT > MIC |
Nephrotoxicity/Neurotoxicity Cmin > 361 mg/L (Piperacillin nephro-/neurotoxicity) Cmin > 20 mg/L (Cefepime neurotoxicity) Cmin > 44.5 mg/L (Meropenem nephro-/neurotoxicity) |
Aminoglycosides
|
AUC-based/Two samples 3 Cmax/MIC/One sample 4 Cmin/One sample 1 |
AUC 80–120 mg h/L Cmax/MIC ≥ 8–10 Cmin <0.5 mg/L Cmin <2.5 mg/L |
Nephrotoxicity/Ototoxicity Cmin> 1 mg/L Cmin> 5 mg/L |
Glycopeptides
|
AUC/MIC/Two samples 5 Cmin/One sample 1 Css/One sample 2 |
AUC (0–24)/MIC ≥ 400 Cmin ≥ 15–20 mg/L 6 Css 20–25 mg/L |
Nephrotoxicity Cmin > 20 mg/L |
| Therapeutic Drug Monitoring Neither Recommended nor Discouraged | |||
| Colistin | Cmin/One sample 1 AUC (0–24)/MIC |
Cmin 2 mg/L Not defined |
Nephrotoxicity Cmin > 2.4 mg/L |
| Fluoroquinolones | AUC/MIC/Two samples 7 Cmax/MIC/One sample 4 |
fAUC0–24/MIC ≥ 80 Cmax/MIC ≥ 8–12 |
Not defined |
6. Other Approaches to Optimize Dosing–Nebulization
The increased prevalence of VAP caused by MDR pathogens, the poor lung penetration of commonly prescribed AM, and the absence of new AM in the pipeline led clinicians to search for alternative approaches to optimize drug dosing in the lung, of which the use of inhaled antibiotics is the most used and studied approach [5][56][57][58].
Nebulized antibiotics have been used with two aims: as an alternative to IV antibiotics and as an adjunctive therapy in addition to IV. The main aim of this strategy is to achieve good antibiotic concentrations at the lung parenchyma, minimizing systemic effects: namely toxicity, antibiotic pressure, and the rate of emergence of MDR pathogens [59]. The use of nebulized antibiotics as an isolated therapy in pneumonia (as an alternative to the IV route) cannot be recommended, since there are no data to support this strategy. Moreover, 10–20% of VAP present secondary bacteremia that would not be adequately treated with this approach [31].
There are also some potential benefits from this approach, namely the decrease in the emergence of MDR pathogens. Since the epithelial lining fluid concentrations attained with nebulization are frequently well above MIC, such high levels in the lung might contribute to decreasing the risk of emergence of drug resistance [60]. Although controversial, this has been suggested in two studies in patients with ventilator-associated tracheobronchitis [59][61] but without robust data from the recent RCTs.
7. Impact of Dosing Strategies on Outcomes
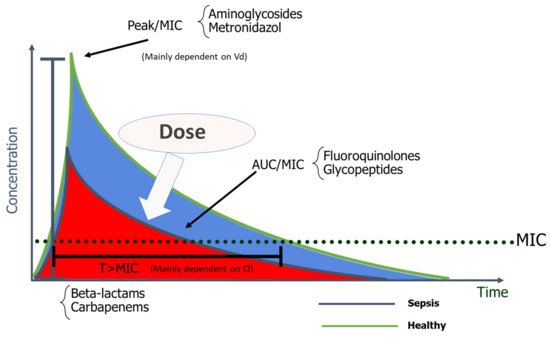
8. Future Directions and Conclusions
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377.
- Goncalves-Pereira, J.; Paiva, J.A. Dose modulation: A new concept of antibiotic therapy in the critically ill patient? J. Crit. Care 2013, 28, 341–346.
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509.
- Ulldemolins, M.; Roberts, J.A.; Lipman, J.; Rello, J. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 2011, 139, 1210–1220.
- Jamal, W.; Al Roomi, E.; AbdulAziz, L.R.; Rotimi, V.O. Evaluation of Curetis Unyvero, a multiplex PCR-based testing system, for rapid detection of bacteria and antibiotic resistance and impact of the assay on management of severe nosocomial pneumonia. J. Clin. Microbiol. 2014, 52, 2487–2492.
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11.
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851.
- Mouton, J.W.; Punt, N.; Vinks, A.A. Concentration-effect relationship of ceftazidime explains why the time above the MIC is 40 percent for a static effect in vivo. Antimicrob. Agents Chemother. 2007, 51, 3449–3451.
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10.
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012, 141, 1327–1336.
- Varghese, J.M.; Roberts, J.A.; Lipman, J. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit. Care Clin. 2011, 27, 19–34.
- Gous, A.; Lipman, J.; Scribante, J.; Tshukutsoane, S.; Hon, H.; Pinder, M.; Mathivha, R.; Verhoef, L.; Stass, H. Fluid shifts have no influence on ciprofloxacin pharmacokinetics in intensive care patients with intra-abdominal sepsis. Int. J. Antimicrob. Agents 2005, 26, 50–55.
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011, 50, 99–110.
- SAFE Study Investigators; Finfer, S.; Bellomo, R.; McEvoy, S.; Lo, S.K.; Myburgh, J.; Neal, B.; Norton, R. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: Analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ 2006, 333, 1044.
- Williams, P.; Cotta, M.O.; Roberts, J.A. Pharmacokinetics/Pharmacodynamics of beta-Lactams and Therapeutic Drug Monitoring: From Theory to Practical Issues in the Intensive Care Unit. Semin Respir Crit. Care Med. 2019, 40, 476–487.
- Goncalves-Pereira, J.; Povoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of beta-lactams. Crit. Care 2011, 15, R206.
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153.
- Andes, D.; Craig, W.A. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: Application to breakpoint determinations. Antimicrob. Agents Chemother. 1998, 42, 2375–2379.
- Mouton, J.W.; Punt, N. Use of the t > MIC to choose between different dosing regimens of beta-lactam antibiotics. J. Antimicrob. Chemother. 2001, 47, 500–501.
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501.
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300.
- Taccone, F.S.; Laterre, P.F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.L.; et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126.
- Pea, F.; Viale, P.; Furlanut, M. Antimicrobial therapy in critically ill patients: A review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 2005, 44, 1009–1034.
- Roberts, J.A.; Pea, F.; Lipman, J. The clinical relevance of plasma protein binding changes. Clin. Pharmacokinet. 2013, 52, 1–8.
- Roger, C.; Louart, B.; Elotmani, L.; Barton, G.; Escobar, L.; Koulenti, D.; Lipman, J.; Leone, M.; Muller, L.; Boutin, C.; et al. An international survey on aminoglycoside practices in critically ill patients: The AMINO III study. Ann. Intensive Care 2021, 11, 49.
- Giamarelloum, H. Aminoglycosides plus beta-lactams against gram-negative organisms. Evaluation of in vitro synergy and chemical interactions. Am. J. Med. 1986, 80, 126–137.
- Giamarellou, H.; Zissis, N.P.; Tagari, G.; Bouzos, J. In vitro synergistic activities of aminoglycosides and new beta-lactams against multiresistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984, 25, 534–536.
- Klastersky, J.; Meunier-Carpentier, F.; Prevost, J.M.; Staquet, M. Synergism between amikacin and cefazolin against Klebsiella: In vitro studies and effect on the bactericidal activity of serum. J. Infect. Dis. 1976, 134, 271–276.
- Paul, M.; Lador, A.; Grozinsky-Glasberg, S.; Leibovici, L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. 2014.
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864.
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111.
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur. Respir. J. 2017.
- Gu, W.J.; Wang, F.; Tang, L.; Bakker, J.; Liu, J.C. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2014, 44, 477–485.
- Tulli, G.; Messori, A.; Trippoli, S.; Marinai, C. Non-inferiority of colistin compared with standard care for the treatment of ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2017, 49, 638–641.
- Florescu, D.F.; Qiu, F.; McCartan, M.A.; Mindru, C.; Fey, P.D.; Kalil, A.C. What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin. Infect. Dis. 2012, 54, 670–680.
- Rotschafer, J.C.; Ullman, M.A.; Sullivan, C.J. Optimal use of fluoroquinolones in the intensive care unit setting. Crit. Care Clin. 2011, 27, 95–106.
- Mihu, C.N.; Rhomberg, P.R.; Jones, R.N.; Coyle, E.; Prince, R.A.; Rolston, K.V. Escherichia coli resistance to quinolones at a comprehensive cancer center. Diagn. Microbiol. Infect. Dis. 2010, 67, 266–269.
- Graffunder, E.M.; Venezia, R.A. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J. Antimicrob. Chemother. 2002, 49, 999–1005.
- Pepin, J.; Saheb, N.; Coulombe, M.A.; Alary, M.E.; Corriveau, M.P.; Authier, S.; Leblanc, M.; Rivard, G.; Bettez, M.; Primeau, V.; et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: A cohort study during an epidemic in Quebec. Clin. Infect. Dis. 2005, 41, 1254–1260.
- Kollef, M.H. Antibiotics for the critically ill: More than just selecting appropriate initial therapy. Crit. Care 2013, 17, 146.
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082.
- Pea, F.; Bertolissi, M.; Di Silvestre, A.; Poz, D.; Giordano, F.; Furlanut, M. TDM coupled with Bayesian forecasting should be considered an invaluable tool for optimizing vancomycin daily exposure in unstable critically ill patients. Int. J. Antimicrob. Agents 2002, 20, 326–332.
- Goncalves-Pereira, J.; Martins, A.; Povoa, P. Pharmacokinetics of gentamicin in critically ill patients: Pilot study evaluating the first dose. Clin. Infect. Dis. 2010, 16, 1258–1263.
- Galar, A.; Munoz, P.; Valerio, M.; Cercenado, E.; Garcia-Gonzalez, X.; Burillo, A.; Sanchez-Somolinos, M.; Juarez, M.; Verde, E.; Bouza, E. Current use of daptomycin and systematic therapeutic drug monitoring: Clinical experience in a tertiary care institution. Int. J. Antimicrob. Agents 2019, 53, 40–48.
- Galar, A.; Valerio, M.; Munoz, P.; Alcala, L.; Garcia-Gonzalez, X.; Burillo, A.; Sanjurjo, M.; Grau, S.; Bouza, E. Systematic Therapeutic Drug Monitoring for Linezolid: Variability and Clinical Impact. Antimicrob. Agents Chemother. 2017, 61.
- Wong, G.; Sime, F.B.; Lipman, J.; Roberts, J.A. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect Dis 2014, 14, 288.
- Dhaese, S.A.M.; Thooft, A.D.J.; Farkas, A.; Lipman, J.; Verstraete, A.G.; Stove, V.; Roberts, J.A.; De Waele, J.J. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: A prospective observational study. J. Crit. Care 2019, 52, 75–79.
- Lipman, J.; Wallis, S.C.; Boots, R.J. Cefepime versus cefpirome: The importance of creatinine clearance. Anesth. Analg. 2003, 97, 1149–1154.
- Goncalves-Pereira, J.; Silva, N.E.; Mateus, A.; Pinho, C.; Povoa, P. Assessment of pharmacokinetic changes of meropenem during therapy in septic critically ill patients. BMC Pharmacol. Toxicol. 2014, 15, 21.
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387.
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596.
- Leisman, D.; Huang, V.; Zhou, Q.; Gribben, J.; Bianculli, A.; Bernshteyn, M.; Ward, M.F.; Schneider, S.M. Delayed Second Dose Antibiotics for Patients Admitted from the Emergency Department with Sepsis: Prevalence, Risk Factors, and Outcomes. Crit. Care Med. 2017, 45, 956–965.
- Lipman, J.; Roberts, J. Does Appropriate Antibiotic Therapy Mean Only Adequate Spectrum and Timing? Crit. Care Med. 2015, 43, 1773–1774.
- Arulkumaran, N.; Routledge, M.; Schlebusch, S.; Lipman, J.; Conway Morris, A. Antimicrobial-associated harm in critical care: A narrative review. Intensive Care Med. 2020, 46, 225–235.
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506.
- Drusano, G.L. What are the properties that make an antibiotic acceptable for therapy of community-acquired pneumonia? J. Antimicrob. Chemother. 2011, 66 (Suppl. S3), iii61–iii67.
- Sweeney, D.A.; Kalil, A.C. Why don’t we have more inhaled antibiotics to treat ventilator-associated pneumonia? Clin. Microbiol. Infect. 2019, 25, 1195–1199.
- Sweeney, D.A.; Kalil, A.C. The last breath for inhaled antibiotics and VAP? Not so fast. Lancet Infect. Dis. 2020, 20, 265–266.
- Palmer, L.B.; Smaldone, G.C. Reduction of bacterial resistance with inhaled antibiotics in the intensive care unit. Am. J. Respir. Crit. Care Med. 2014, 189, 1225–1233.
- Schreiber, M.P.; Shorr, A.F. Inhaled antibiotics for the treatment of pneumonia. Curr. Opin. Pulm. Med. 2019, 25, 289–293.
- Palmer, L.B.; Smaldone, G.C.; Chen, J.J.; Baram, D.; Duan, T.; Monteforte, M.; Varela, M.; Tempone, A.K.; O’Riordan, T.; Daroowalla, F.; et al. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit. Care Med. 2008, 36, 2008–2013.
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86.
- Roberts, J.A. Using PK/PD to optimize antibiotic dosing for critically ill patients. Curr. Pharm Biotechnol. 2011, 12, 2070–2079.
- Roberts, J.A.; Roberts, M.S.; Semark, A.; Udy, A.A.; Kirkpatrick, C.M.; Paterson, D.L.; Roberts, M.J.; Kruger, P.; Lipman, J. Antibiotic dosing in the ’at risk’ critically ill patient: Linking pathophysiology with pharmacokinetics/pharmacodynamics in sepsis and trauma patients. BMC Anesthesiol. 2011, 11, 3.
- Richter, D.C.; Frey, O.; Rohr, A.; Roberts, J.A.; Koberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011.
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741.
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558.
- Scheetz, M.H.; Lodise, T.P.; Downes, K.J.; Drusano, G.; Neely, M. The case for precision dosing: Medical conservatism does not justify inaction. J. Antimicrob. Chemother. 2021.
- Carlier, M.; Carrette, S.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int. J. Antimicrob. Agents 2014, 43, 470–473.
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351.




