In recent years, the knowledge of pharmacokinetics and pharmacodynamics, drug dosing, therapeutic drug monitoring, and antimicrobial resistance in the critically ill patients has greatly improved, fostering strategies to optimize therapeutic efficacy and to reduce toxicity and adverse events. Nonetheless, delivering adequate and appropriate antimicrobial therapy is still a challenge, since pathogen resistance continues to rise, and new therapeutic agents remain scarce.
1. Introduction
One of the recommendations from the Surviving Sepsis Campaign (SSC) is antibiotic therapy in the first hour [
1]. This is a key element for successful sepsis management. However, for this to be effective, several decisions must be addressed simultaneously when prescribing antimicrobials (AM): (1) AM choices should be adequate, covering the most probable pathogens; (2) they should be administered in the appropriate dose, (3) by the correct route, and (4) using the correct mode of administration to achieve successful concentration at the infection site.
It is well known that inadequate empirical antibiotic therapy is associated with poor outcomes. However, there are scarce data concerning the impact of inadequate dosing on outcomes of critically ill patients [
2,
3]. Moreover, patients with sepsis and septic shock present an increased risk of underdosing, increased volume of distribution (Vd), increased clearance, risk of overdosing, and risk of renal and hepatic failure. In addition, we are facing infections frequently caused by pathogens with higher minimum inhibitory concentrations, consequently increasing the risk of inadequate dosing. In order to be effective, AM dosing should be optimized to quickly attain bactericidal concentrations at the infection site. To optimize the AM exposure of pathogens, it is also fundamental to consider drug penetration in different organs both in health and disease [
4,
5].
2. Pharmacokinetic and Pharmacodynamic Characteristics of Antimicrobials
The therapeutic window of a drug is defined according to previously studied dose–response relationships which will also determine the limits of safe concentration and dosage. The dose and duration of dosing intervals of AM are determined according to their pharmacokinetic/pharmacodynamic (PK/PD) properties [6]. However, in critical illness, multiple underlying derangements provoke pathophysiological alterations that change the PK/PD of drugs and therefore provoke dynamic changes in drug concentration.
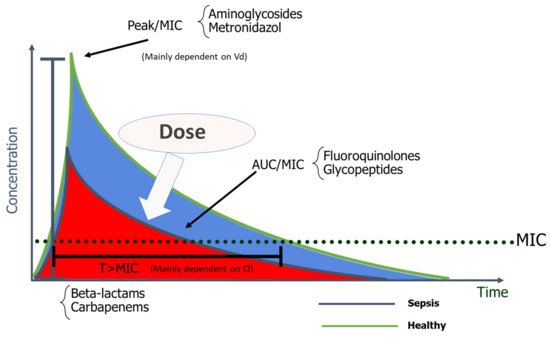
Antimicrobials are classified according to their dose–response relationships into the following PK/PD groups: time-dependent, concentration-dependent, and concentration-dependent with time-dependence [
6,
12]. The effect of time-dependent AM, such as β-lactams, depends on the cumulative percentage of time over 24 h by which the free AM concentration exceeds the MIC (%fT > MIC). The killing rate does not improve if concentration greatly exceeds the MIC [
13]. In concentration-dependent AM, such as aminoglycosides (AG), their effect depends on the peak concentration divided by the MIC (Peak/MIC). The higher the AM concentration, the greater the extent and rate of bactericidal activity [
10]. Optimal Peak/MIC targets for AG will be further discussed in this review. The effect of concentration-dependent drugs with time-dependence, such as fluoroquinolones and glycopeptides, is determined by the AUC 0–24 h divided by the MIC, and specific targets, as will be further discussed, depend on the AM [
6,
10].
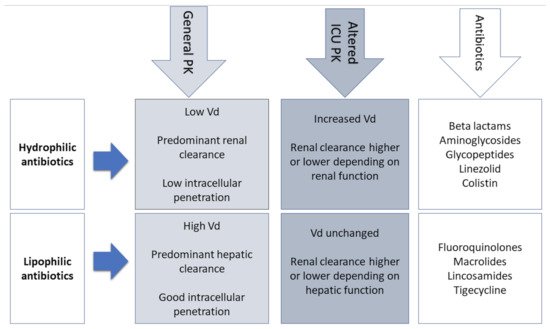
The physicochemical properties of AM should also integrate the choice of appropriate dosing (Figure 1).
Figure 1. Physicochemical properties of antibiotics. Vd—Volume of distribution; PK—Pharmacokinetics.
In the ICU patient, traditional dosing strategies will most likely be insufficient to achieve the desired PK/PD targets of maximal AM activity. Therefore, an individualized approach considering specific MICs and regimens most likely to attain PK/PD goals can provide reasonable solutions.
3. PK Changes in Critically Ill Patients
Critically ill patients present important pathophysiological changes that significantly modify the PK of antimicrobials [
7]. In septic shock, blood flow of gastrointestinal tract and subcutaneous tissue are severely reduced and shunted to vital organs such as the brain and heart, compromising reliable drug absorption with administration via these routes. As a result, intravenous administration of AM is always recommended in patients with sepsis and septic shock [
1,
7].
Patients with sepsis and septic shock present a significant fluid shift from the intravascular compartment to interstitial space due to endothelial damage and increased capillary leak. This leak results in severe hypotension requiring aggressive intravenous volume resuscitation that further increases the Vd, the eventual simultaneous prescription of vasopressors, and development of organ failures such as circulatory shock and renal failure (
Table 1). For these reasons, hydrophilic AM (aminoglycosides, β-lactams, glycopeptides, and lipopeptides) with an extracellular distribution need a higher loading dose to achieve therapeutic concentrations [
3,
18]. On the other hand, the Vd of lipophilic antibiotics is not significantly influenced by these changes and does not require dose adjustments [
19].
Table 1. Volume of distribution of ICU antibiotics.
Antibiotics that Stay in Extracellular Fluid
(Vd < 0.3 L/kg) |
Drugs that Distribute into Total Body Water
(Vd 0.7–1 L/kg) |
Drug with High Distribution to Tissues
(Vd > 1 L/kg) |
- -
-
Aminoglycosides
- -
-
Beta-lactams
- -
-
Penicillins
- -
-
Cephalosporins
- -
-
Carbapenems
- -
-
Daptomycin
|
- -
-
Clindamycin
- -
-
Linezolid
- -
-
Metronidazole
- -
-
Vancomycin
|
- -
-
Colistin
- -
-
Fluoroquinolones
- -
-
Macrolides
- -
-
Azithromycin
- -
-
Clarithromycin
- -
-
Tigecyline
|
Another important factor that can influence the Vd of AM is the modification in protein binding. Since albumin is the main plasma-binding protein for many AM (e.g., cefazolin, ceftriaxone, ertapenem, and daptomycin), its decreased concentration in septic patients has a direct impact on the PK of antibiotics [20]. With low plasma albumin, there is an increase of the unbound antibiotic, increasing its Vd and clearance, and leading to lower and probably suboptimal AM concentrations toward the end of dosing intervals. For these reasons, therapeutic drug monitoring (TDM) should include an adjustment for low albumin levels or a direct measurement of free drug levels [21].
4. Antibiotics in the ICU
4.1. Β-Lactams
The β-lactams’ broad spectrum of AM activity and low toxicity profiles unsurprisingly render them first-line options in serious infections, namely Gram-negative bacilli (GNB) infections, and the most commonly prescribed AM in critical care [14,31].
Β-lactams are generally hydrophilic, with low Vd, moderate to low protein binding, and essentially renal excretion [15]. In vivo animal studies have clearly shown that β-lactams are characterized by a slow continuous kill, in other words, time-dependent bactericidal activity [32,33]. Consequently %fT > MIC is the optimal PK/PD parameter for β-lactams, with the recommended interval of 40–70% [15,34], varying according to the AM and underlying pathogens [35]. This time-dependent effect is independent of peak values and little post-antibiotic effect exists, except for carbapenems [36]. Since β-lactams have short or no post-antibiotic effect, when AM concentration falls below the MIC at the infection site, residual pathogens can rapidly regrow [16]. Furthermore, frequent Vd and Cl alterations accentuate the risk of suboptimal drug concentrations in the face of critical illness [15]. For example, with hypoalbuminemia, highly protein-bound β-lactams such as ceftriaxone, ertapenem, flucloxacillin, and oxacillin will present increased free fractions [37].
4.2. Aminoglycosides
Aminoglycosides are frequently prescribed as empirical therapy regimens in septic ICU patients, namely when suspicion of GNB infection prevails [
61]. Furthermore, recent guidelines recommend combination therapy in septic shock [
1]. The rationale for combination therapy originated from in vitro findings of synergistic bactericidal activity with certain combination therapies in the context of
Pseudomonas aeruginosa and other GNB infections [
62,
63,
64]. However, in a recent metanalysis comparing β-lactam monotherapy with β-lactam/aminoglycoside combination therapy, evidence regarding non-neutropenic septic patients does not show a mortality benefit with combination therapy [
65].
Aminoglycosides are hydrophilic, with low Vd and drug clearance proportional to GFR [
15]. Extended-interval dosing of a high, single dose is recommended in GNB infections.
4.3. Glycopeptides
Vancomycin
Most published data regarding vancomycin dosing and TDM are retrospective observational or PK/PD assessments, with few published RCT. Since the 2009 guidelines on the treatment of serious methicillin-resistant Staphylococcus aureus (MRSA) infections, new light has been shed regarding the efficacy and safety of previous recommendations [
75]. Issues such as dosing strategies in obese patients, safety profiles in daily dosages exceeding 3 g, continuous infusion strategies, and renal failure are some examples where insufficient data precluded adequate coverage. Moreover, existing recommendations of exposure effectiveness are based mainly on studies of MRSA bacteriemia, with fewer studies of pneumonia and endocarditis. Nevertheless, much controversy around vancomycin dosing and TDM still exists [
75].
Vancomycin is hydrophilic, has a low Vd, and elimination is mainly renal. Altered Vd and drug clearance, namely ARC, in the critically ill may lead to low drug exposure [
15].
4.4. Colistin
Current guidelines for ventilator-associated pneumonia (VAP) recommend empirical combination therapy with colistin and another antipseudomonal AM in ICUs where carbapenem-resistant (CR) GNB are highly prevalent [
93,
94]. Recent meta-analyses evaluated the efficacy and safety of colistin for VAP caused by MDR GNB and found it to have similar efficacy and safety as seen with β-lactams. However, multiple limitations of the studies included call into question the strength of these findings [
95,
96,
97].
4.5. Fluoroquinolones
The AM spectrum of fluoroquinolones includes GNB, Gram-positive, and with some, also anaerobic coverage, with popular use since the 1980s [
107]. However, over the years, GNB resistance has drastically increased, with susceptibility rates less than 70% for agents such as
Escherichia coli,
Pseudomonas aeruginosa, and
Proteus mirabilis [
107,
108]. Moreover, the frequent and inappropriate use of this class has been associated with
Clostridium difficile infection outbreaks and the emergence of MRSA [
109,
110]. The issues related to fluoroquinolone resistance have led to their infrequent use as first-line AM in the ICU setting where GNB such as
Pseudomonas aeruginosa,
Acinetobacter baumannii, and
Stenotrophomonas maltophilia are often fluoroquinolone-resistant. However, when used in the ICU setting, fluoroquinolones should be administered at maximum doses (levofloxacin 750 mg every 24 h; ciprofloxacin 400 mg every 8 h) [
107].
5. Strategies to Optimize Dosing
There is increasing evidence that front-line antibiotic inappropriateness is common and may have significant impact on the outcome of patients with severe infections and septic shock [
31,
36]. Large spectrum AM as well as combination therapy have both been proposed as strategies to enlarge antibacterial spectrum and improve patient outcomes [
1]. However, appropriate spectrum of antibiotic therapy may be insufficient if adequate exposure is missed [
3,
118]. Early achievement of adequate antibiotic concentration is of paramount importance when treating patients with septic shock.
Promoting high peak concentration for concentration-dependent antibiotics (e.g., aminoglycosides), which is concentrating the daily dose on only one time-point, or, on the contrary, promoting long exposure time, for time-dependent antibiotics (e.g., penicillins), with prolonged or continuous infusions, was proposed to optimize therapeutic success [
122]. However, these strategies may be flawed in the presence of PK changes: high concentration peaks may be toxic or, in contrast, inadequately low (especially in patients with a changing Vd), and the time between doses may not be enough to achieve adequate trough concentration. In addition, in the presence of altered clearance, the concentration of time-dependent antibiotics may always be under the adequate target or, alternately, it may accumulate and lead to toxic concentrations [
3]. Conventional or nomogram-guided dosing may easily fail to achieve the intended target concentration. This has been demonstrated for vancomycin [
123], aminoglycosides [
72], daptomycin [
124], linezolid [
125], and also β-lactams [
126,
127]. Consequently, an interest in TDM has grown (
Table 2).
Table 2. Pharmacokinetic and pharmacodynamic characteristics of common antibiotics in intensive care medicine [
15].
| Antimicrobial Class |
Monitoring/
Sampling |
PK/PD Target |
Toxicity
Threshold |
| Therapeutic Drug Monitoring Recommended |
Beta-lactams
- -
-
Penicillins
- -
-
Cephalosporins
- -
-
Carbapenems
|
Cmin/One sample 1
Css (continuous infusion)/One sample 2 |
100% fT> MIC
Css > MIC
50–100% fT > MIC
45–100% fT > MIC
50–100% fT > MIC |
Nephrotoxicity/Neurotoxicity
Cmin > 361 mg/L (Piperacillin nephro-/neurotoxicity)
Cmin > 20 mg/L (Cefepime neurotoxicity)
Cmin > 44.5 mg/L (Meropenem nephro-/neurotoxicity) |
Aminoglycosides
- -
-
Gentamicin
- -
-
Amikacin
|
AUC-based/Two samples 3
Cmax/MIC/One sample 4
Cmin/One sample 1 |
AUC 80–120 mg h/L
Cmax/MIC ≥ 8–10
Cmin <0.5 mg/L
Cmin <2.5 mg/L |
Nephrotoxicity/Ototoxicity
Cmin> 1 mg/L
Cmin> 5 mg/L |
Glycopeptides
- -
-
Vancomycin
|
AUC/MIC/Two samples 5
Cmin/One sample 1
Css/One sample 2 |
AUC (0–24)/MIC ≥ 400
Cmin ≥ 15–20 mg/L 6
Css 20–25 mg/L |
Nephrotoxicity
Cmin > 20 mg/L |
| Therapeutic Drug Monitoring Neither Recommended nor Discouraged |
| Colistin |
Cmin/One sample 1
AUC (0–24)/MIC |
Cmin 2 mg/L
Not defined |
Nephrotoxicity
Cmin > 2.4 mg/L |
| Fluoroquinolones |
AUC/MIC/Two samples 7
Cmax/MIC/One sample 4 |
fAUC0–24/MIC ≥ 80
Cmax/MIC ≥ 8–12 |
Not defined |
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9071401