Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elena Vialkova | + 3227 word(s) | 3227 | 2021-07-06 05:40:36 | | | |
| 2 | Vivi Li | Meta information modification | 3227 | 2021-07-09 05:06:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vialkova, E. Microwave Irradiation and Wastewater. Encyclopedia. Available online: https://encyclopedia.pub/entry/11855 (accessed on 07 February 2026).
Vialkova E. Microwave Irradiation and Wastewater. Encyclopedia. Available at: https://encyclopedia.pub/entry/11855. Accessed February 07, 2026.
Vialkova, Elena. "Microwave Irradiation and Wastewater" Encyclopedia, https://encyclopedia.pub/entry/11855 (accessed February 07, 2026).
Vialkova, E. (2021, July 09). Microwave Irradiation and Wastewater. In Encyclopedia. https://encyclopedia.pub/entry/11855
Vialkova, Elena. "Microwave Irradiation and Wastewater." Encyclopedia. Web. 09 July, 2021.
Copy Citation
Every year, the human impact on the world’s water sources becomes more pronounced. One of the triggers to this increase is the use of ineffective wastewater and sludge treatment systems. Recently, the number of studies of microwave processing in handling liquid municipal and industrial waste has increased. This paper discusses heat treatment, change in properties, decomposition of substances, removal of metals, demulsification, pyrolysis, biogas processing, disinfection, and other topics.
microwave irradiation (MW)
wastewater (WW)
wastewater/sewage sludge (WWS)
treatment technology
MW installations/devices/reactors
1. Introduction
The size of global urban water consumption can be estimated based on the total population of Earth living in urban environments, which is more than 3.4 billion people, and the average water consumption per human of 499 m3 per year [1]. While accurate data on the amount of wastewater (WW) generated around the world is not available, using a calculated method based on the standard of water use and the percentage of irreversible losses (roughly 30–35 per cent) can help us assume that more than 1100 billion m3 of wastewater and 0.1–0.05% of that amount—wastewater sludge (WWS)—is treated and disposed of annually in urbanised settlements. Solving high-quality WW and WWS treatment issues leads to a decrease in the anthropogenic impact on water and land resources. New methods and technologies, such as electromagnetic microwave irradiation (MW), may help make a breakthrough in this field.
Microwave radiation is electromagnetic ultrahigh-frequency radiation, including decimeter, centimetre, and millimetre ranges of radio waves with a frequency of 0.3 GHz to 300 GHz, corresponding to a wavelength from 1 m to 1 mm [2][3]. High-intensity microwaves are commonly used for contactless heating of objects, including stoves for cooking food, metallurgical industry heat treatment installations, and medical devices for treating veins. People actively involve microwaves of a particular range (frequency from 1 to 100 GHz) in radiolocation. MW reactors are increasingly adopted to neutralise various solid and liquid wastes produced by people and industrial enterprises [4][5][6].
Microwave irradiation is confidently entering the wastewater (WW) and sludge (WWS) treatment technology in the twenty-first century, primarily as an alternative to conditional heating [4]. MW is a well-known heating and drying process used both for domestic and industrial purposes.
This method has several advantages over conventional electric heating, including non-contact heating, instant and rapid process with a high degree of uniformity, and precise heating, which induces dipolar oscillations and ionic conductivity in the medium [7][8]. In 2011, researchers conducted a comprehensive study of the existing state of MW technology for WW treatment [9]. According to this report, using MW to decompose contaminants has numerous advantages. For example, selectivity and reaction rate increase while reaction time, activation energy, equipment size, and waste parameters decrease.
These benefits are primarily due to the thermal and nonthermal effects of microwave irradiation. Many transformations with a beneficial impact on the structure and properties of water occur in the aquatic environment under the thermal influence of MW: increased dissolution of substances, coagulation and demulsification of pollution, activation of various chemical reactions (including oxidation of organic matter), and degradation of toxins; the disinfecting effect of MW is well known [10].
2. Heating and Thermal Treatment
MW has become increasingly common as a thermal method for treating wastewater and sediments in recent years, owing to its rapid and selective heating [9][10][11][12][13][14][15]. The thermal effect of MW [16] describes how ultrahigh-frequency energy can be consumed by microwave absorbers and dissipated as thermal energy. For many environments, including water solutions, microwave heating with dielectric losses is typical [13].
Water is a positive-charged molecule (or dipole) with a negative-charged opposite end. Dipolar polarization occurs due to intermolecular inertia, responsible for most of the microwave heating observed in liquids. The rapid change in the electric field of microwave radiation causes a rotation of dipoles. At the same time, the rate at which the dipole rotates (reverses) cannot accurately correlate to the rate at which the electric field shifts direction. It induces “internal friction” between water molecules, which leads to direct and very uniform heating of the reaction mixture. However, reflections and refractions at local boundaries between phases lead to the appearance of so-called “hot spots” and the effect of “overheating”, which has been extensively discussed by researchers [15][17][18][19][20][21].
Figure 1 presents the schematic diagram of microwave action [3][17], illustrating the advantages and scope of application of microwave processing. Microwave heating penetrates the liquid and creates the rapidly changing field: dipoles (water molecules) continuously react attempting to align in the field, which generates heat; heat is uniformly distributed throughout the water.
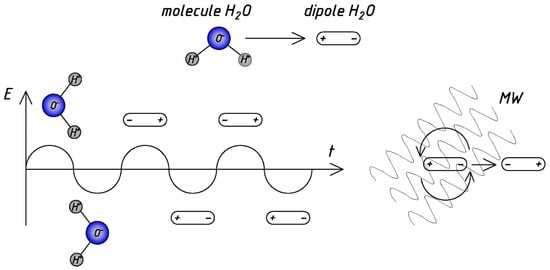
Figure 1. The scheme of mechanism of the MW water heating.
Under the influence of MW, several parameters such as strength, frequency, duration, treatment temperature, and sample volumes [9][14][22][23][24][25][26][27] can influence the efficiency of pollutant decomposition and mineralisation of wastewater and sediments. It is confirmed in the materials [25][26][27], which use the Netherlands, Kenya, China, and other countries as examples of MW-heating of faecal sludge (Figure 2).
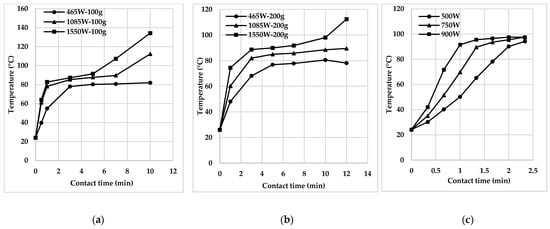
As a rule, the efficiency of the MW system tends to rise with the increase of power and time of microwave irradiation [22]. It is due to the release of extra heat, contributing to the rapid movement of water molecules. In addition, increased time and power of irradiation amplify the decomposition of various contaminants in the water environment [9].
In some cases, the efficiency of the MW system is reduced at very high temperatures by evaporating water and increased viscosity of the substance by overheating. Thus, it is necessary to determine the optimal power and reaction temperature for decomposing a particular target pollutant [9][14][17][18][19][20][21].
It should be noted that the technological and economic efficiency of MW heating for the water environment is currently actively explored by contrasting it to other methods of heating and processing [14][23][24]. The Department of Water Supply and Sanitation (Industrial University of Tyumen, Russia) laboratory has carried out several experiments related to the MW-heating of wastewater sludge [14][24]. Firstly, a comparison is made between microwave and electric heating. The distinctive feature of microwave heating is its thermal effect, which is volumetric and does not involve thermal diffusion from the surface into the material, as conventional heating does, which explains its high thermoset reaction rates. According to observations, ultrahigh-frequency irradiation of liquid sewage sludge has a rapid thermal effect: samples of sludge with a 50–300 mL volume started to boil within one to two minutes (Figure 3) [14].
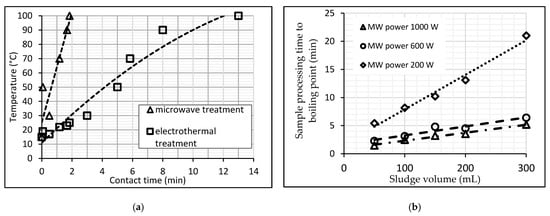
Figure 3. (a) Comparison of two methods of WWS-heating. (b) Boiling time dependence of the WWS on the sample volume at a constant power MW.
Figure 3a compares the heating curves of sewage sludge (mixture of the raw sludge and activated sludge) in two different ways, with the rate of heating the sludge to a given temperature using microwaves being four to six times faster than the usual heating on an electric stove. In addition, in the process of microwave irradiation, an improvement in the sedimentation and compaction of WWS was obtained by 13–15% compared to traditional convective heating to the same temperature [14].
Secondly, the maximum time for MW treatment of WWS samples to reach boiling point [24] was determined experimentally. Figure 3b illustrates dependency t = f (V) at constant power MW based on the experience data. Obviously, the higher the microwave processing power, the faster the sludge samples reach the boiling point. At MW 200 W, the heating rate of sewage sludge is 3.7–4.0 times lower than at MW 1000 W and 3–2.8 times lower than at MW 600 W. Heating sludge with a power of 1000 W is 1.2 times more effective than heating with 600 W. Rapid and voluminous MW-heating of wastewater and sediments entails other positive effects discussed below.
3. Decontamination (Disinfection)
In the last century, the biophysical impact of the MW field on the viability and other properties of bacteria was discovered [28][29]. The sterilising efficiency of the MW field produced by the GZ–10A generator when irradiated for 10 min, for example, was used to assess the biological effect of microwaves on microorganisms [30]. The bacteria’s viability was determined by the number of colonies developed within two days on the breeding ground. As a result, researchers discovered a bactericidal effect of pulsed and continuous microwaves on Escherichia coli and staphylococcus cultures.
Experiments on the influence of centimetre waves on the growth of Escherichia coli M–17 in a continuous mode (frequency 10.6 GHz, PPM = 0.1–5.5 MW/cm2) are presented in the paper [31]. According to the material results, microwave radiation has a harmful effect on escherichia coli (n = 10) at a power of 130 W for five minutes [32]. Water heating and disinfection systems were invented and patented in the 1970s and 1980s [33][34].
The modern use of MW for wastewater disinfection is based on earlier studies [35][36]. Less often it is mentioned that sewage sludge is also disinfected during microwave irradiation [14][26][27][37][38][39][40][41][42]. Water disinfection usually occurs at a power of MW from 300 W and higher (frequency 2.45 GHz) when heated from 45 to 100 °C. Therefore, the processing time depends on the sample volume and the MW heating power. This knowledge is very relevant concerning the further disposal of such liquid municipal waste. For example, faecal sludge formed in public toilets was treated using a laboratory microwave installation (MW) [26][27].
Total bacterial inactivation was achieved in 30–240 min after sewage sludge treatment in a special MW reactor [41]. According to findings in [42], high–level disinfection for enterococci and salmonella is possible to achieve in 9.5 min at MW energy consumption of 580 W∙s/g and temperature 72 °C. In some studies [43][44], microwave irradiation proved to effectively reduce the bacterial content of sewage sludge prior to anaerobic digestion. In addition, a high degree of removal of faecal coliforms in the sediment is recorded in [45] (the content of 2.66 logs or less).
Similarly, researchers [46] confirmed that a single pretreatment with microwaves resulted in a 50% reduction of bacteria C.Perfringens. Furthermore, according to the article [14], the microwave treatment of a mixture of sewage sludge can achieve 99% decontamination from all pathogenic bacteria subject to control.
The MW technology can be further investigated for potential expansion as a rapid treatment alternative for faecal effluents and sediments in emergencies [26][27], such as a pandemic.
4. Decomposition of Organic Substances
Organic pollution of natural and wastewater is a source of concern for scientists and environmentalists worldwide, as these contaminants have a detrimental impact on the natural environment, human life, and health. Approximately 3000 different organic contaminants have been identified [47][48] and classified into three groups: (1) organic substances of natural origin, (2) synthetic organic pollutants, and (3) chemicals reformed in water as a result of its purification. Many organic pollutants of the second and third groups are toxins and carcinogens [49]. Therefore, the international community is looking for creative, highly efficient advanced oxidative water treatment technologies that involve various pollutant exposure processes to address this problem.
In order to increase the performance of WW treatment from different contaminants and minimise reaction time, microwave exposure should be combined with oxidising agents OX (MW + OX), adsorbents activated carbon AC (MW + OX + AC), catalysts carbon C (MW + OX + C), and advanced oxidation processes with the addition of UV irradiation such as photo-Fenton (MW + OX + C + UV), direct photolysis using an electrodeless discharge lamp EDL (MW+OX+EDL), and photocatalysis using TiO2 photocatalyst (MW + OX + UV + TiO2) [50][51][52][53][54][55][56][57][58][59][60][61][62].
The review data were summarised reasonably well in the papers [9][63]. Other studies of the efficacy of MW oxidation of organic compounds under various treatment conditions are seen in Table 1 [22][51][52][53][54][55][56][57][58][59][60][61][62].
Table 1. The efficiency of MW oxidation of organic substances in WW.
| Type of an Organic Substance | Sample Volume | Concentration | Oxidizing Agent, Catalyst, pH | MW Power | MW Duration, Temperature | Effect | [Ref./No] |
|---|---|---|---|---|---|---|---|
| Sample was only MW-treated | |||||||
| Ammonia (laboratory installation) | 100 mL | 0.5–12 g/L | Air 1 L/min pH = 11 |
750 W | 3 min 80 °C |
D * 98.4–96.1% | [53] |
| Ammonia (pilot plant) | 28,000 mL | 2.4–11 g/L | Air 30 L/min pH = 11.6–12 |
4.8 kW | 60 min 80–100 °C |
D * 80% | [53] |
| With an addition of the oxidizer: MW + OX | |||||||
| Naphthalene Disulfonic Acid | 10 mL | 1.0 mmol/L | H2O2 | 300 W | 20 min 30 min 80 °C |
D * 90% M ** 50% | [54] |
| Dimethoate (phosphoric compound) | No Data | 0.1 mmol/L | K2S2O8 pH = 6.8 |
750 W | 4 min 100 °C |
D * 100% | [22] |
| Perflurooctanic acid | 50 mL | 0.25 mmol/L | Na2S2O8 | 800 W | 240 min 60–130 °C |
D * 99.3% M ** 74.3% |
[56] |
| The photo-Fenton process: MW + OX + C + UV | |||||||
| Polyacrylamide (PAA) | No Data | 150 mg/L | H2O2/AC pH = 3 |
70 W 490 W |
6 min | D * 20% D * 80% |
[51] |
| Pesticides (dimethoate, triazophos, malathion) | 1000 mL | 6.11–31.65 mg/L | H2O2 Fe2+; pH = 5 |
80 W | 120 min 25 °C |
M ** 72.1% | [52] |
| Direct photolysis: MW + OX + EDL | |||||||
| Phenol | 50 mL | 200 mg/L | H2O2 | 1000 W | 9 min 30 min 50 °C |
D * 90% M ** 95% |
[57] |
| Atrazine | 50 mL | 50 mg/L | pH = 6.3 | 900 W | 30 min 30 °C |
D * 100% | [58] |
| Photocatalysis: MW + OX + UV + TiO2 | |||||||
| Methylene Blue (aromatic compound) | 50 mL | 100 mg/L | TiO2 load pH = 7 |
900 W | 15 min 100 °C |
D * 96% M ** 50% |
[59] |
| 2,4–D chlorophenoxyacetic herbicide | 10 mL | 0.04 mmol/L | TiO2 load pH = 4.9 |
700 W | 20 min 200 °C |
D * 100% | [60] |
| Bisphenol A (Endocrine disruptor) | 30 mL | 0.1 mM | TiO2 load pH = 6.7 |
1500 W | 90 min 150 °C |
M ** 100% | [61] |
| Phenol | 50 mL | 10 mg/L | TiO2/AC | 900 W | 30 min 1000 °C |
D * 87% | [62] |
| Atrazine | 50 mL | 20 mg/L | TiO2 nanotubes pH = 8.1 |
900 W | 5 min 20 min |
D * 100% M ** 98.5% |
[62] |
D *—destruction efficiency; M **—mineralisation efficiency.
The addition of microwaves to oxidising agents accelerates the oxidation of organic compounds due to dipolar polarisation. For each pollutant, the form of oxidising agents, necessary doses, and reaction conditions (including temperature, strength, and treatment period MW) are calculated separately [54][56]. Microwave capacity ranges from 300 W to 900 W, temperature ranges from 20 °C to 130 °C, and processing time ranges from three minutes to one hour, depending on sample volume. Thus, the pollutant characteristics and their resistance to temperature and chemical factors affect the variance of parameter values.
Various catalysts, such as ferromagnetic metal, transition metal oxides, various types of activated carbons, and others, are applied to the water to increase the MW oxidation of organic compounds. The catalysts can be added to the water in two different ways: a suspension accompanied by sedimentation or a fixed filter plate. At the same time, the removal efficiency for a wide range of organic pollutants is about 85–100% [9].
A promising technology for the degradation of organic pollutants, even from the stage of mineralisation, is considered to be catalytic oxidation by moist air (CWAO) under conditions of high temperature (180–315 °C) and pressure (2–25 MPa), with the addition of catalysts [9]. The photo-Fenton process is based on the use of the Fenton reagent, that is, a mixture of Fe2+ salt (catalyst) and hydrogen peroxide (oxidiser) in combination with ultraviolet irradiation (UV). The study [9] provides research on the decomposition and mineralisation of different organic pollutants and reveals that compared to Fenton and photo-Fenton processes without MW, the decomposition rate of various pollutants increases by at least 50 times.
Electrodeless discharge lamp (EDL) use eliminates the issue of electrode destruction in a conventional mercury-based UV lamp. EDL consists of a glass tube—a plasma chamber filled under reduced pressure with argon and excitable matter (Hg, HgI2, Cd, I2, KI, P, Se, and S) and generating UV radiation under the action of MW (direct photolysis–MWDP). Together with microwaves and oxidising agents, MWDP is considered the most effective by many authors [9][57][58][64][65].
Microwave photocatalysis emerged to speed up and deepen organic carbon oxidation and mineralisation reactions, preventing secondary corrosion and iron-containing sediment production. The TiO2 semiconductor photocatalyst is commonly used in this method in grains, nanoporous films, and nanotubes. In addition, the TiO2 composite catalyst supported on activated carbon TiO2/AC shows good results [9].
The authors [9][13][55][66] consider the most critical factors influencing the efficiency of decomposition and mineralisation of organic pollutants based on comprehensive experimental experience with microwaves for the removal of organic pollutants: microwave power (W), irradiation time (min), and exposure temperature (°C).
In complex treatment, the optimum dosages of oxidising agents and catalysts, pH values, and air supply parameters to the device must be determined. In addition to the influencing factors mentioned above, the light intensity and amount of oxygen in the solution are applied to photocatalysis and microwave photolysis reactions [60]. Thus, each specific organic contamination must determine the optimal values of these parameters of microwave exposure, depending on the required efficiency of destruction and mineralisation.
One urgent task of applying innovative oxidation methods is to optimise energy consumption, particularly when using microwaves. Hence, according to the authors [9], the least energy-intensive methods are MW+K and MW+UV+TiO2; the most energy–intensive is water treatment using only microwaves [50]. On the other hand, MW radiation in the pulsed mode significantly saves energy [9][50].
The prospect of using MW in wastewater treatment is more justified in the presence of challenging organic substances that are not biodegradable.
Microwave irradiation has several practical uses, including the oxidation of synthetic dyes in the wastewater of industrial establishments in the textile, leather, cosmetic, food, paper, pharmaceutical, and other industries. In the presence of oxidants and catalysts, a 65–100% reduction in dye concentration can be achieved in 1.5–210 min at MW power of 150–900 W [63][64][65][67][68][69][70].
There is confirmation of successful MW oxidation of naphthenic acid, typical for industrial wastewater of oil-producing enterprises [71]. MW can be used for complex oxidation of wastewater containing ammonia [53], phosphorous compounds [55], phenols [9][52], pesticides [51], PAA [52], medical preparations [9], and other elements.
5. Biogas Processing
European and Asian experience in managing liquid municipal waste gives preference to the anaerobic treatment of wastewater and sediments to produce biogas. The maximum number of biogas plants operate in China—approximately fifteen million, and India—about ten million. The construction of biogas plants is actively developing in Europe, especially in Germany, with more than 9000 stations. Only 7% of the biogas produced by these enterprises goes to the gas pipelines. The rest is used for the manufacturer’s needs. In the future, 10–20% of the natural gas used in the country can be replaced with biogas [72][73][74].
The biogas market is growing much more slowly in countries with natural gas resources. For example, only about 200 biogas plants operate on agricultural waste in the United States [74]. In Russia, the production of biogas is implemented in only a few WWTP [75].
Today, the anaerobic treatment of wastewater and sediments to produce biogas guarantees fuel and energy savings. However, there are problems related to the quality of the treated material, the provision of conditions for the stability of the biodegradation of organic matter, and the explosion hazard of biogas production [76][77].
Microwaving is a novel thermal pretreatment process for sludges that improves digestion efficiency and, under certain conditions, can intensify the gas output by 15–32% due to the solubilisation and hydrolysis of organic substances [78][79][80][81][82][83][84]. Previous studies have shown that MW pretreatment is more effective for sludge with a high concentration of solid particles [80][81]. It is also proved that in the thermophilic fermentation mode, the yield of biogas from the MW-treated sediment is higher than in the mesophilic mode [78]. The results of modern studies based on [78][85] are presented in Table 2.
Table 2. Intensification of the biogas output during the MW treatment of WWS.
| Sediment | MW Power and Frequency | Temperature | Sample Processing Conditions | Description of Results | [Ref./No] |
|---|---|---|---|---|---|
| Domestic wastewater sludge mixture, ratio 48:52 | 1250 W 2.45 GHz |
96 °C | 500 mL samples heated in a home MW furnace to the boiling point, then subjected to anaerobic digestion in laboratory reactors for 5–18 days; biogas output recorded. | With thermophilic fermentation, the gas output increased by 17–26%. |
[78] |
| Dehydrated WWS | 1200 W 2.45 GHz |
80–160 °C | Samples heated and kept at a set temperature for 1 min, then cooled for 25 min. Heating speed 7.5 °C/min. Further, the samples were subjected to anaerobic digestion in laboratory reactors for 5–20 days; biogas output was recorded. | Maximal biogas output: At 160 °C- on the fifth day of the fermentation process. At 120 °C on the tenth day. | [84] |
| Sludge mixture | 300–600 W 2.45 GHz |
no data | Microwave pre-treatments were carried out in a semi–pilot MW unit in which the flow rate varied in the range of 5–60 L/h. Next, anaerobic digestion of the nitrogen-treated sludge mixture was carried out at a temperature of 37 degrees. | The biogas production improved by 174–210% (depending on the MW power and irradiated energy) | [85] |
References
- Priroda, S.U. Nature, Ecology and Environment. Available online: (accessed on 4 May 2021).
- Harris, P.W.; McCabe, B.K. Review of pre-treatments used in anaerobic digestion and their potential application in high-fat cattle slaughterhouse wastewater. Appl. Energy 2015, 155, 560–575.
- Tyagi, V.; Lo, S.-L. Microwave irradiation: A sustainable way for sludge treatment and resource recovery. Renew. Sustain. Energy Rev. 2013, 18, 288–305.
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577.
- Elagroudy, S.; El-Gohary, F. Microwave Pretreatment of Mixed Sludge for Anaerobic Digestion Enhancement. Environ. Eng. 2013, 5, 105–111.
- Kichigin, V.; Samara State Technical University; Zemlyanova, M.; Vyalkova, E.; Tumen State Industrial University. Study of the Possibility of Using Microwave Radiation for the Treatment of Liquid Municipal Waste. Urban Constr. Arch. 2018, 8, 44–49.
- Mudhoo, A.; Sharma, S.K.; Sharma, S.K. Microwave Irradiation Technology in Waste Sludge and Wastewater Treatment Research. Crit. Rev. Environ. Sci. Technol. 2011, 41, 999–1066.
- Wiesbrock, F.; Hoogenboom, R.; Schubert, U.S. Microwave-Assisted Polymer Synthesis: State-of-the-Art and Future Perspectives. Macromol. Rapid Commun. 2004, 25, 1739–1764.
- Remya, N.; Lin, J.-G. Current status of microwave application in wastewater treatment—A review. Chem. Eng. J. 2011, 166, 797–813.
- Gole, V.L.; Gogate, P.R. Degradation of brilliant green dye using combined treatment strategies based on different irradiations. Sep. Purif. Technol. 2014, 133, 212–220.
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal Microwave Effects Revisited: On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry. J. Org. Chem. 2008, 73, 36–47.
- Yang, L.; Chen, Z.; Yang, J.; Liu, Y.; Wang, J.; Yu, Y.; Gao, X. Removal of volatile fatty acid in landfill leachate by the microwave-hydrothermal method. Desalination Water Treat. 2013, 52, 4423–4429.
- Wei, R.; Wang, P.; Zhang, G.; Wang, N.; Zheng, T. Microwave-responsive catalysts for wastewater treatment: A review. Chem. Eng. J. 2020, 382, 122781.
- Vialkova, E.; Zemlyanova, M.; Danilov, O. Energy efficiency in municipal waste treatment. MATEC Web Conf. 2018, 170, 04020.
- Hidaka, H.; Saitou, A.; Honjou, H.; Hosoda, K.; Moriya, M.; Serpone, N. Microwave-assisted dechlorination of polychlorobenzenes by hypophosphite anions in aqueous alkaline media in the presence of Pd-loaded active carbon. J. Hazard. Mater. 2007, 148, 22–28.
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284.
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97.
- Clark, D.E.; Folz, D.C.; West, J.K. Processing materials with microwave energy. Mater. Sci. Eng. A 2000, 287, 153–158.
- Horikoshi, S.; Serpone, N. Photochemistry with microwaves: Catalysts and environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 96–110.
- Baghurst, D.R.; Mingos, D.M.P. Superheating effects associated with microwave dielectric heating. J. Chem. Soc. Chem. Commun. 1992, 674–677.
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224.
- Zhang, L.; Guo, X.; Yan, F.; Su, M.; Li, Y. Study of the degradation behaviour of dimethoate under microwave irradiation. J. Hazard. Mater. 2007, 149, 675–679.
- Akbari, S.; Nour, A.; Jamari, S.; Rajabi, A. Demulsification of Water-in-Crude Oil Emulsion via Conventional Heating and Microwave Heating Technology in Their Optimum Conditions. Aust. J. Basic Appl. Sci. 2016, 10, 66–74.
- Vialkova, E.; Zemlyanova, M.; Fugaeva, A. Treatment and utilization of liquid communal waste in the cities. MATEC Web Conf. 2018, 212, 03005.
- Yu, Q.; Lei, H.; Li, Z.; Li, H.; Chen, K.; Zhang, X.; Liang, R. Physical and chemical properties of waste-activated sludge after microwave treatment. Water Res. 2010, 44, 2841–2849.
- Mawioo, P.M.; Rweyemamu, A.; Garcia, H.A.; Hooijmans, C.M.; Brdjanovic, D. Evaluation of a microwave based reactor for the treatment of blackwater sludge. Sci. Total Environ. 2016, 548–549, 72–81.
- Mawioo, P.M.; Hooijmans, C.M.; Garcia, H.A.; Brdjanovic, D. Microwave treatment of faecal sludge from intensively used toilets in the slums of Nairobi, Kenya. J. Environ. Manag. 2016, 184, 575–584.
- Makarov, P.O. Biophysical Bases of the Action of Ultraviolet and Ultrasonic Radiation and Ultrahigh-Frequency Electromagnetic Field. Lectures on Biophysics; LSU Publishing House: Leningrad, Russia, 1968; pp. 209–233.
- Devyatkov, N.D. Influence of Millimeter-band Electromagnetic Radiation on Biological Objects. Sov. Phys. Uspekhi 1974, 16, 568–569.
- Belitsky, B.I. Study of the effect of the microwave field on microorganisms in pulse and continuous mode. Biophysics 1982, 27, 923–927.
- Panasenko, V.I. The Effect of a Powerful EMF with a Frequency of 2375 Mhz on Microorganisms. Biological Effect of Electromagnetic Fields; USSR: Pushchino, Russia, 1982; pp. 26–27.
- Benjamin, E.; Reznik, A.; Williams, A.L. Mathematical Models for Conventional and Microwave Thermal Deactivation of Enterococcus Faecalis, Staphylococcus aureus and Escherichia coli. Cell Mol. Biol. 2007, 53, 42–48.
- Rosenberg, H.C. System for Purifying Liquids. U.S. Patent 4013558A, 22 March 1977.
- Zabolotskij, L.L.; Klimarev, S.I.; Lobanov, A.G. Device for Disinfection and Heating of Aqueous Media. Patent SU1139439A1, 1985.
- Klimarev, S.I.; Grigorev, A.A.; Sinyak, Y.E. Method for Liquids Disinfection and Heating, and Device for Its Implementation. Russian Patent RU2627899, 14 August 2017.
- Ahmedova, O.O.; Stepanov, S.F.; Soshinov, A.G.; Bahtiarov, K.H. Increase of Efficiency of Local Treatment Facilities of Sewage for the Account of Application of the Combined Electrophysical Methods of Influence. Mod. Probl. Sci. Educ. 2009, 5, 56–60.
- Parmar, H.; Asada, M.; Kanazawa, Y.; Asakuma, Y.; Phan, C.M.; Pareek, V.; Evans, G.M. Influence of Microwaves on the Water Surface Tension. Langmuir 2014, 30, 9875–9879.
- Wong, T.W.; Iskhandar, A.; Kamal, M.; Jumi, S.J.; Kamarudin, N.H.; Zin, N.Z.M.; Salleh, N.H.M. Effects of Microwave on Water and Its Influence on Drug Dissolution. Prog. Electromagn. Res. C 2009, 11, 121–136.
- Zemlyanova, M.V. Intensification of Household Wastewater Sludge Treatment by Ultra-High Frequency Electromagnetic Radiation. Ph.D. Thesis, Samarskiy Gosudarstvennyy Arkhitekturno-Stroitel’nyy Universitet, Samara, Russia, 2015. Available online: (accessed on 3 May 2021).
- Liu, J.; Wei, Y.; Li, K.; Tong, J.; Wang, Y.; Jia, R. Microwave-acid pretreatment: A potential process for enhancing sludge dewaterability. Water Res. 2016, 90, 225–234.
- Rao, B.; Su, X.; Lu, X.; Wan, Y.; Huang, G.; Zhang, Y.; Xu, P.; Qiu, S.; Zhang, J. Ultrahigh pressure filtration dewatering of municipal sludge based on microwave pretreatment. J. Environ. Manag. 2019, 247, 588–595.
- Karlsson, M.; Carlsson, H.; Idebro, M.; Eek, C. Microwave Heating as a Method to Improve Sanitation of Sewage Sludge in Wastewater Plants. IEEE Access 2019, 7, 142308–142316.
- Ara, E.; Sartaj, M.; Kennedy, K. Effect of microwave pre-treatment of thickened waste activated sludge on biogas production from co-digestion of organic fraction of municipal solid waste, thickened waste activated sludge and municipal sludge. Waste Manag. Res. 2014, 32, 1200–1209.
- Kenge, A.; Liao, P.H.; Lo, K.V. Solubilization of municipal anaerobic sludge using microwave-enhanced advanced oxidation process. J. Environ. Sci. Health Part A 2009, 44, 502–506.
- Hong, S.M.; Park, J.K.; Teeradej, N.; Lee, Y.O.; Cho, Y.K.; Park, C.H. Pretreatment of Sludge with Microwaves for Pathogen Destruction and Improved Anaerobic Digestion Performance. Water Environ. Res. 2006, 78, 76–83.
- Kuglarz, M.; Karakashev, D.B.; Angelidaki, I. Microwave and thermal pretreatment as methods for increasing the biogas potential of secondary sludge from municipal wastewater treatment plants. Bioresour. Technol. 2013, 134, 290–297.
- Worthington, P. Organic Micropollutants in the Aqueous Environment. In Studies in Environmental Science; Pawlowski, L., Alaerts, G., Lacy, W.J., Eds.; Chemistry for Protection of the Environment 1985; Elsevier: Amsterdam, The Netherlands, 1986; Volume 29, pp. 235–244.
- Randtke, S.J. Organic Contaminant Removal by Coagulation and Related Process Combinations. J. Am. Water Work. Assoc. 1988, 80, 40–56.
- Kuzubova, L.I.; Morozov, S.V. Organic contaminant of drinking water. Ecology. World Lit. Rev. Ser. 1993, 1, 1–167.
- Garcia-Costa, A.L.; Zazo, J.A.; Casas, J.A. Microwave-assisted catalytic wet peroxide oxidation: Energy optimization. Sep. Purif. Technol. 2019, 215, 62–69.
- Cheng, G.; Lin, J.; Lu, J.; Zhao, X.; Cai, Z.; Fu, J. Advanced Treatment of Pesticide-Containing Wastewater Using Fenton Reagent Enhanced by Microwave Electrodeless Ultraviolet. BioMed Res. Int. 2015, 2015, 1–8.
- Wang, N.; Sun, X.; Zhao, Q.; Wang, P. Treatment of polymer-flooding wastewater by a modified coal fly ash-catalysed Fenton-like process with microwave pre-enhancement: System parameters, kinetics, and proposed mechanism. Chem. Eng. J. 2021, 406, 126734.
- Lin, L.; Chen, J.; Xu, Z.; Yuan, S.; Cao, M.; Liu, H.; Lu, X. Removal of ammonia nitrogen in wastewater by microwave radiation: A pilot-scale study. J. Hazard. Mater. 2009, 168, 862–867.
- Ravera, M.; Buico, A.; Gosetti, F.; Cassino, C.; Musso, D.; Osella, D. Oxidative degradation of 1,5-naphthalenedisulfonic acid in aqueous solutions by microwave irradiation in the presence of H2O2. Chemosphere 2009, 74, 1309–1314.
- Jung, S.C. The microwave-assisted photo-catalytic degradation of organic dyes. Water Sci. Technol. 2011, 63, 1491–1498.
- Lee, Y.-C.; Lo, S.-L.; Chiueh, P.-T.; Chang, D.-G. Efficient decomposition of perfluorocarboxylic acids in aqueous solution using microwave-induced persulfate. Water Res. 2009, 43, 2811–2816.
- Han, D.-H.; Cha, S.-Y.; Yang, H.-Y. Improvement of oxidative decomposition of aqueous phenol by microwave irradiation in UV/H2O2 process and kinetic study. Water Res. 2004, 38, 2782–2790.
- Ta, N.; Hong, J.; Liu, T.; Sun, C. Degradation of atrazine by microwave-assisted electrodeless discharge mercury lamp in aqueous solution. J. Hazard. Mater. 2006, 138, 187–194.
- Hong, J.; Sun, C.; Yang, S.-G.; Liu, Y.-Z. Photocatalytic degradation of methylene blue in TiO2 aqueous suspensions using microwave powered electrodeless discharge lamps. J. Hazard. Mater. 2006, 133, 162–166.
- Horikoshi, S.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination technique: VI. A simple modified domestic microwave oven integrating an electrodeless UV-Vis lamp to photodegrade environmental pollutants in aqueous media. J. Photochem. Photobiol. A Chem. 2004, 161, 221–225.
- Horikoshi, S.; Tokunaga, A.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination method: VII. Thermal/non-thermal effects in the microwave-assisted photocatalyzed mineralization of bisphenol-A. J. Photochem. Photobiol. A Chem. 2004, 162, 33–40.
- Zhanqi, G.; Shaogui, Y.; Na, T.; Cheng, S. Microwave assisted rapid and complete degradation of atrazine using TiO2 nanotube photocatalyst suspensions. J. Hazard. Mater. 2007, 145, 424–430.
- Verma, P.; Samanta, S.K. Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 2018, 16, 969–1007.
- Zhang, X.; Wang, Y.; Li, G.; Qu, J. Oxidative decomposition of azo dye C.I. Acid Orange 7 (AO7) under microwave electrodeless lamp irradiation in the presence of H2O2. J. Hazard. Mater. 2006, 134, 183–189.
- Hong, J.; Ta, N.; Yang, S.-G.; Liu, Y.-Z.; Sun, C. Microwave-assisted direct photolysis of bromophenol blue using electrodeless discharge lamps. Desalination 2007, 214, 62–69.
- Wang, N.; Wang, P. Study and application status of microwave in organic wastewater treatment—A review. Chem. Eng. J. 2016, 283, 193–214.
- Ju, Y.; Yang, S.; Ding, Y.; Sun, C.; Gu, C.; He, Z.; Qin, C.; He, H.; Xu, B. Microwave-enhanced H2O2-based process for treating aqueous malachite green solutions: Intermediates and degradation mechanism. J. Hazard. Mater. 2009, 171, 123–132.
- Yang, S.; Wang, P.; Yang, X.; Wei, G.; Zhang, W.; Shan, L. A novel advanced oxidation process to degrade organic pollutants in wastewater: Microwave-activated persulfate oxidation. J. Environ. Sci. 2009, 21, 1175–1180.
- Zhang, X.; Li, G.; Wang, Y. Microwave assisted photocatalytic degradation of high concentration azo dye Reactive Brilliant Red X-3B with microwave electrodeless lamp as light source. Dye. Pigment. 2007, 74, 536–544.
- Anshuman, A.; Saremi-Yarahmadi, S.; Vaidhyanathan, B. Enhanced catalytic performance of reduced graphene oxide–TiO2 hybrids for efficient water treatment using microwave irradiation. RSC Adv. 2018, 8, 7709–7715.
- Mishra, S.; Meda, V.; Dalai, A.K.; Headley, J.V.; Peru, K.M.; McMartin, D.W. Microwave treatment of naphthenic acids in water. J. Environ. Sci. Health Part A 2010, 45, 1240–1247.
- Kütük, M.A.; Aksoy, M. A Case Study on Sewage Sludge Incineration Plant: Gaski. In Proceedings of the Second International Conference on Water, Energy and the Environment, Kusadası, Turkey, 21–24 September 2013; pp. 1–6.
- Blagojevic, V.; Sustersic, V.; Bozickovic, S.; Veselin, B.; Vanja, Š.; Siniša, B. Pyrolysis and gasification in the process of sewage sludge treatment. Zast. Mater. 2017, 58, 305–312.
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340.
- Khramenkov, S.V.; Pakhomov, A.N.; Khrenov, K.E.; Streltsov, S.A.; Khamidov, M.G.; Belov, N.A. Utilization of Biogas and Creation of Autonomous Sources of Power Supply at Treatment Facilities. Water Supply Sanit. Tech. 2010, 10-1, 48–53.
- Fericelli, P.D. Comparison of Sludge Treatment by Gasification vs. Incineration. In Proceedings of the Ninth LACCEI Latin American and Caribbean Conference (LACCEI’2011), Medellín, Colombia, 3–5 August 2011; p. 10.
- Danilovich, D.A. Crisis of competence in the design of wastewater treatment facilities. Best Available Technol. Water Supply Sanit. 2018, 4, 5–13.
- Coelho, N.M.G.; Droste, R.L.; Kennedy, K.J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res. 2011, 45, 2822–2834.
- Hong, S.-M. Enhancement of Pathogen Destruction and Anaerobic Digestibility Using Microwaves. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 2002.
- Eskicioglu, C.; Kennedy, K.J.; Droste, R.L. Enhancement of batch waste activated sludge digestion by microwave pretreatment. Water Environ. Res. 2007, 79, 2304–2317.
- Eskicioglu, C.; Droste, R.L.; Kennedy, K.J. Performance of Anaerobic Waste Activated Sludge Digesters after Microwave Pretreatment. Water Environ. Res. 2007, 79, 2265–2273.
- Toreci, I.; Kennedy, K.J.; Droste, R.L. Effect of High-Temperature Microwave Irradiation on Municipal Thickened Waste Activated Sludge Solubilization. In Proceedings of the 11th Conference on Process Integration, Modeling and Optimization for Energy Saving and Pollution Reduction (PRES), Prague, Czech Republic, 24–28 August 2008.
- Toreci, I.; Kennedy, K.J.; Droste, R.L. Evaluation of continuous mesophilic anaerobic sludge digestion after high temperature microwave pretreatment. Water Res. 2009, 43, 1273–1284.
- Mehdizadeh, S.N.; Eskicioglu, C.; Bobowski, J.; Johnson, T. Conductive heating and microwave hydrolysis under identical heating profiles for advanced anaerobic digestion of municipal sludge. Water Res. 2013, 47, 5040–5051.
- Haranghy, L.; Kertesz, S.Z.; Vereb, G.; Laszlo, Z.S.; Vagvolgyi, A.; Jakoi, Z.; Czupy, I.; Hodur, C.; Rakhely, G.; Beszedes, S. Intensification of the biodegradation of wastewater sludge by microwave irradiation. Geosci. Eng. 2020, 8, 322–333. Available online: (accessed on 3 May 2021).
More
Information
Subjects:
Construction & Building Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
04 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




