Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Su-Kiat Chua | + 1416 word(s) | 1416 | 2021-06-29 10:25:02 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chua, S. LCZ696 in Patients with Hypertension. Encyclopedia. Available online: https://encyclopedia.pub/entry/11852 (accessed on 07 February 2026).
Chua S. LCZ696 in Patients with Hypertension. Encyclopedia. Available at: https://encyclopedia.pub/entry/11852. Accessed February 07, 2026.
Chua, Su-Kiat. "LCZ696 in Patients with Hypertension" Encyclopedia, https://encyclopedia.pub/entry/11852 (accessed February 07, 2026).
Chua, S. (2021, July 09). LCZ696 in Patients with Hypertension. In Encyclopedia. https://encyclopedia.pub/entry/11852
Chua, Su-Kiat. "LCZ696 in Patients with Hypertension." Encyclopedia. Web. 09 July, 2021.
Copy Citation
Hypertension is a well-known and modifiable risk factor for cardiovascular disease worldwide. However, the management of hypertension remains suboptimal throughout the world. LCZ696 (Sacubitril/valsartan) was approved for the treatment of heart failure. Several randomized control trials showed that LCZ696 achieved the target blood pressure control without significant adverse fffects. This meta-analysis including randomized control trials to precisely determine the effectiveness and safety of LCZ696 for the treatment of high aterial pressure.
LCZ696
ambulatory systolic blood pressure
hypertension
1. Introduction
Hypertension is a well-known and modifiable risk factor for cardiovascular disease worldwide. A study showed that the number of patients will increase to 1.5 billion by 2025 [1]. However, the management of hypertension remains suboptimal throughout the world [2][3]. Sacubitril/valsartan (LCZ696), a first-in-class angiotensin receptor neprilysin inhibitor, showed superior benefits over enalapril in the PARADIGM-HF trial[4] and was approved for the treatment of heart failure with reduced ejection fraction. Neprilysin inhibition results in blood pressure reduction via natriuresis, vasodilatation, and inhibition of the renin-angiotensin-aldosterone system and sympathetic activity[5]. The dual inhibition of neprilysin and the angiotensin receptor has shown complementary effects in blood pressure reduction. Omapatrilat resulted in superior effects on systolic blood pressure and pulse pressure reduction than enalapril[6], but was not approved due to the unacceptable rate of angioedema.
In recent years, several randomized controlled trials (RCTs) have attempted to compare the antihypertensive efficacy and safety of LCZ696 in hypertensive patients[7][8][9][10][11][12][13][14][15][16]. Most of these prospective and double-blind clinical trials showed that LCZ696 achieved the target blood pressure better without significant adverse effects. In addition, several meta-analyses have concluded that LCZ696 may reduce arterial pressure more efficaciously than placebo, particularly the angiotensin receptor blocker (ARB), without increasing overall adverse events[17][18][19].
2. Enrollment of Studies
The flow diagram of the study selection is shown in Figure 1. In total, we identified 287 studies. Of these, 217 were deemed irrelevant after title and abstract screening, and 70 were assessed for eligibility using the full text. Of the 70 studies, 44 were excluded from the analyses after reading the title and abstracts. In the remaining 26 studies, 16 studies were excluded because of the study design, and the outcomes have not been published. Finally, 10 studies were included in the quantitative synthesis. The 10 studies were conducted in a number of different countries, including the U.S., Spain, Germany, U.K., Japan, Taiwan, and China[7][8][9][10][11][12][13][14][15][16]. A total of 5931 patients were randomized to receive either LCZ696 (at doses ranging from 100 to 400 mg per day) or a comparator drug (olmesartan) in five studies[8][11][13][14], valsartan in three studies[7][10][12], amlodipine in one study[9], and placebo in one study. The duration of the studies ranged from 4 to 52 weeks. The trial design, treatment strategies, and safety and efficacy outcomes of the ten included RCTs are summarized in Table 1. All included RCTs were judged to be at a low risk of bias (supplementary Figure S1). Two authors, HFH and LCC assessed the risk of bias of all included trials. The kappa value was 0.78, p = 0.01.
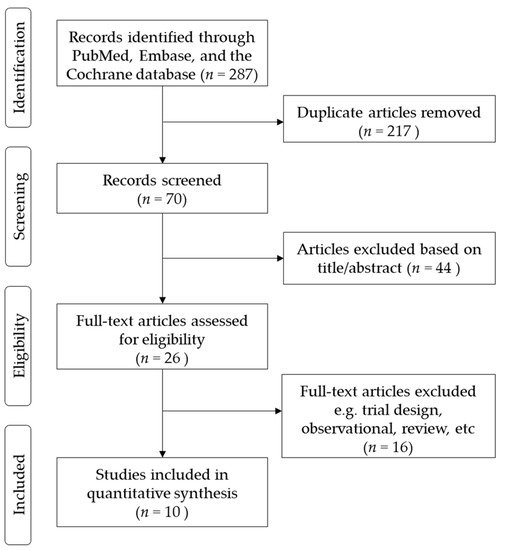
Figure 1. Flow diagram of included studies.
Table 1. Characteristics of the included trials.
| Author, Year | Race | Patients’ Characteristic | Age, Years | Number, (Female/Male) | LCZ696 Dose | Comparison Therapy | Observation Periods | Severe Adverse Events | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Ruilope LM et al., 2010 [7] | not restricted | Mild-to-moderate essential hypertension | 18–75 | 1328 patients (568/760) | 100 mg, 200 mg, 400 mg | Valsartan 80 mg, 160 mg, 320 mg |
8 weeks | Nil | 3/3 |
| Kario K et al., 2014 [8] | only Asians | mild-to-moderate essential hypertension | ≥18 | 389 patients (114/275) | 100 mg, 200 mg, 400 mg | Placebo | 8 weeks | Nil | 2/3 |
| Wang JG et al., 2017 [9] | only Asians | mean clinic SBP 145–180mmHg | ≥18 | 266 patients (110/156) | 200 mg | Amlodipine 5 mg | 8 weeks | Nil | 3/2 |
| Izzo JI et al., 2017 [10] | not restricted | mild-to-moderate systolic hypertension | ≥18 | 907 patients (412/495) | 400 mg | Valsartan 320 mg | 8 weeks | 1 angioedema and 1 CV death in LCZ696 group | 3/3 |
| Schmieder RE et al., 2017 [11] | not restricted | essential hypertension Stage 1 and 2 | ≥18 | 114 patients (37/77) | 400 mg | Olmesartan 40 mg | 52 weeks | NA | 2/2 |
| Wang TD et al., 2017 [12] | only Asians | patient with salt-sensitive hypertension | ≥18 | 72 patients (26/46) | 400 mg | Valsartan 320 mg | 4 weeks | Nil | 2/2 |
| Williams B et al., 2017 [13] | not restricted | msSBP 140–179 mm Hg and PP > 60 mm Hg | ≥60 | 454 patients (217/237) | 400mg | Olmesartan 40 mg | 12 weeks | NA | 3/3 |
| Supasyndh et al., 2017 [16] | only Asians | elderly Asian (≥65 years) | ≥65 | 588 patients (294/294) | 100 mg, 200 mg, 400 mg | Olmesartan 10 mg, 20 mg, 40 mg |
14 weeks | Nil | 3/3 |
| Cheung et al., 2018 [15] | not restricted | mild-to-moderate hypertension |
≥18 | 375 patients (183/192) | 200 mg | Olmesartan 20 mg | 8 weeks | Nil | 2/3 |
| Hou Y et al., 2019 [14] | only Asians | mild-to-moderate hypertension |
≥18 | 1438 patients (682/756) | 200 mg, 400 mg | Olmesartan 20 mg | 8 weeks | 1 Angioedema in each group |
3/3 |
CV, cardiovascular; msSBP, mean systolic blood pressure in sitting position; PP, pulse pressure.
3. Efficacy of the Antihypertensive Effect of Sacubitril/Valsartan (LCZ696)
Both the msSBP and msDBP were calculated in all included studies. The systolic and diastolic blood pressure levels significantly decreased from the baseline after LCZ696 therapy (Figure 2). The pooled weight mean difference (WMD) demonstrated that the blood pressure reductions achieved with LCZ696 were more profound than those found after therapy with olmesartan, valsartan, or placebo. Compared with the comparator therapy (olmesartan in five studies, valsartan in three studies, or amlodipine in one study) or placebo, LCZ696 showed a significant reduction in msSBP with WMD at −6.52 mmHg (95% confidence interval (CI): −8.57 to −4.47; p < 0.001); while the WMD for msDBP was −3.32 mmHg (95% CI: −4.57 to −2.07; p < 0.001). Thereby, evidencing that LCZ696 has greater antihypertensive efficacy with respect to angiotensin receptor blockers or placebo in hypertensive patients at 24–52 weeks.
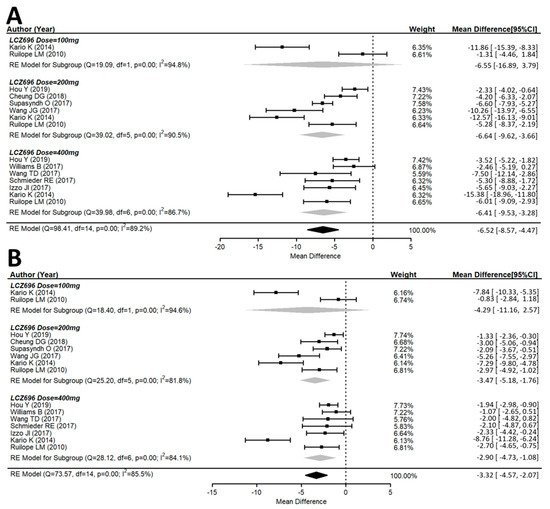
Figure 2. Forest plot of (A) msSBP and (B) msDBP. Comparisons of LCZ696 with a control group. msSBP, mean sitting systolic blood pressure; msDBP, mean sitting diastolic blood pressure.
A total of ten studies explored the maSBP and maDBP from the baseline. These studies showed that LCZ696 is more efficacious than placebo in terms of reducing ambulatory systolic and diastolic blood pressure. Compared with the placebo therapy, LCZ696 showed a significant reduction in maSBP with WMD = −7.08 mmHg (95% CI: −10.48 to −3.68; p < 0.001), and maDBP with WMD = −3.57 mmHg, (95% CI: −5.71 to −1.44, p < 0.001) (Figure 3).
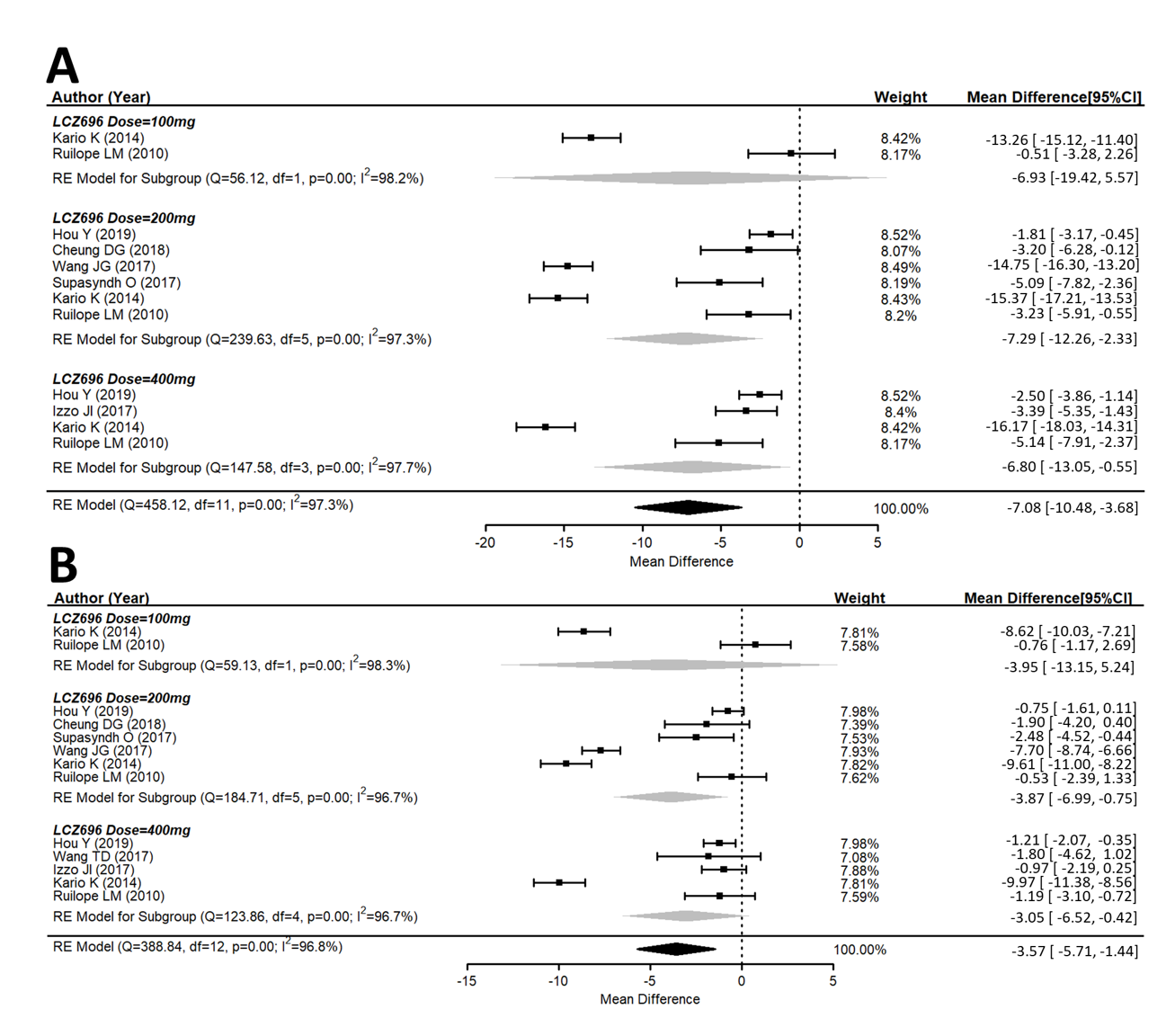
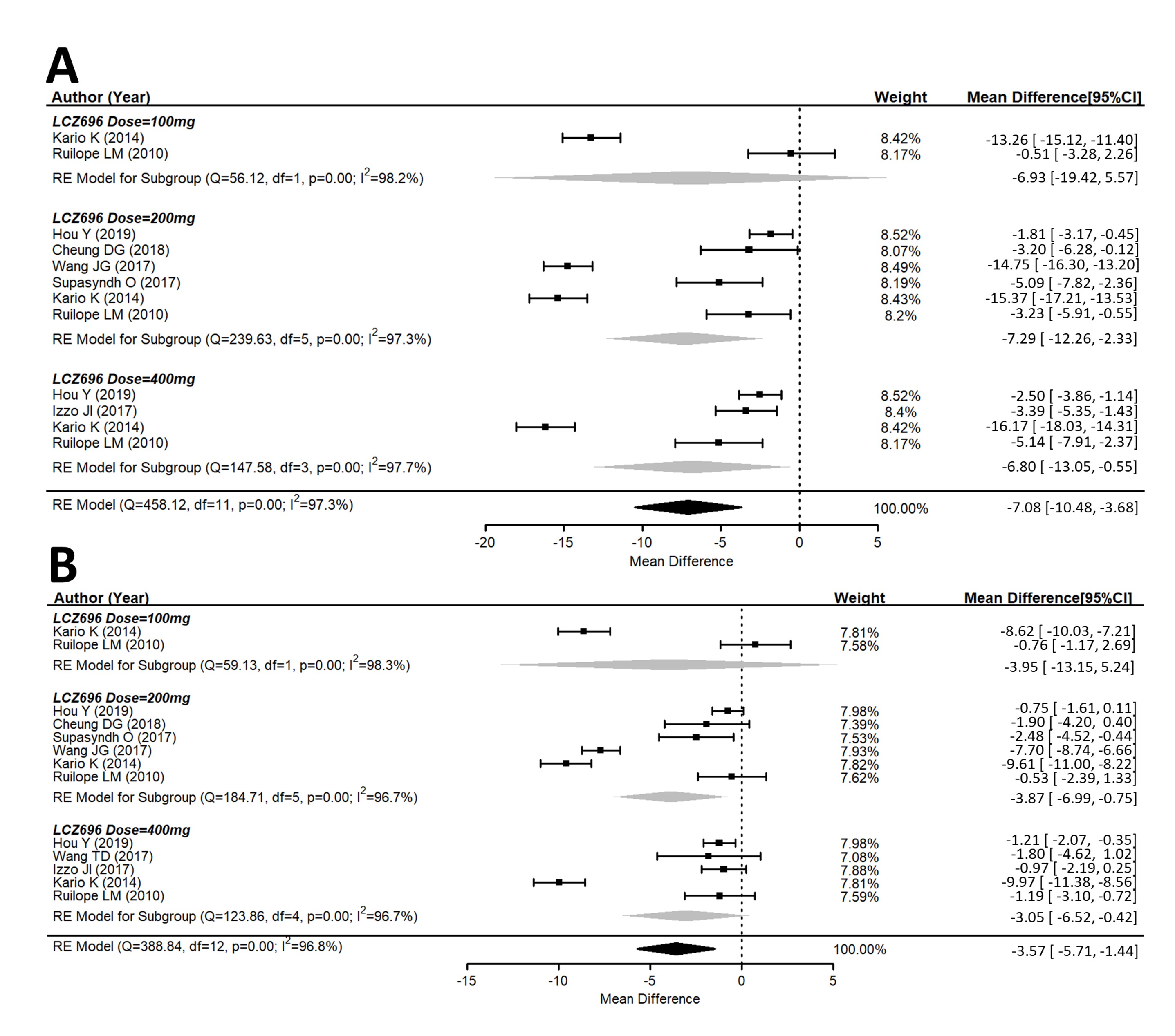
Figure 3. Forest plot of (A) maSBP and (B) maDBP. Comparisons of LCZ696 with a control group. maSBP, mean ambulatory systolic blood pressure; maDBP, mean ambulatory diastolic blood pressure.
4. Effects of Different Doses of Sacubitril/Valsartan (LCZ696) Versus the Placebo Group
In the 100 mg dose of LCZ696 with comparators, the result did not show a difference in the msSBP reduction with WMD at −6.55 mmHg (95% CI: −16.89 to 3.79; p = 0.21). In the 200 mg dose of LCZ696 with comparators, the result showed a significant reduction in msSBP with WMD at −6.64 mmHg (95% CI: −9.62 to −3.66; p < 0.001). In the 400 mg dose of LCZ696 with comparators, the result showed a significant reduction in msSBP with WMD at −6.41 mmHg (95% CI: −9.53 to −3.28; p < 0.001) (Figure 2). In the 100 mg dose of LCZ696 with comparators, the result showed no difference in msDBP reduction with WMD at −4.29 mmHg (95% CI: −11.16 to 2.57; p = 0.21). In the 200 mg dose of LCZ696 with comparators, the result showed a significant reduction in msDBP with WMD at −3.47 mmHg (95% CI: −5.18 to −1.76; p < 0.001). In the 400 mg dose of LCZ696 with comparators, the result showed a significant reduction in msDBP with WMD at −2.90 mmHg (95% CI: −4.73 to −1.08; p < 0.01) (Figure 2).
Similar observations were also seen for maSBP and maDBP (Figure 3A,B).
5. Adverse Effects of Sacubitril/Valsartan (LCZ696)
Several drug-related AEs were reported after therapy in nine studies. The pooled data showed no significant difference between the AE rates of LCZ696 and the placebo (OR = 1.02, 95% CI: 0.83–1.27, p = 0.90) (Figure 4). In subgroups, 100 mg of LCZ696 showed an odds ratio of 0.94 (95% CI: 0.49–1.81, p = 0.86); 200 mg of LCZ696 showed an odds ratio of 1.12 (95% CI: 0.89–1.41, p = 0.34); while 400 mg of LCZ696 had an odds ratio of 1.06 (95% CI: 0.86–1.32, p = 0.57). There was also no significant difference between all subgroups according to different LCZ696 doses. The commonly reported AEs in the LCZ696 groups were nasopharyngitis, headache, dizziness, upper respiratory tract infection, diarrhea, and hyperuricemia. All of which were minimal and mild.
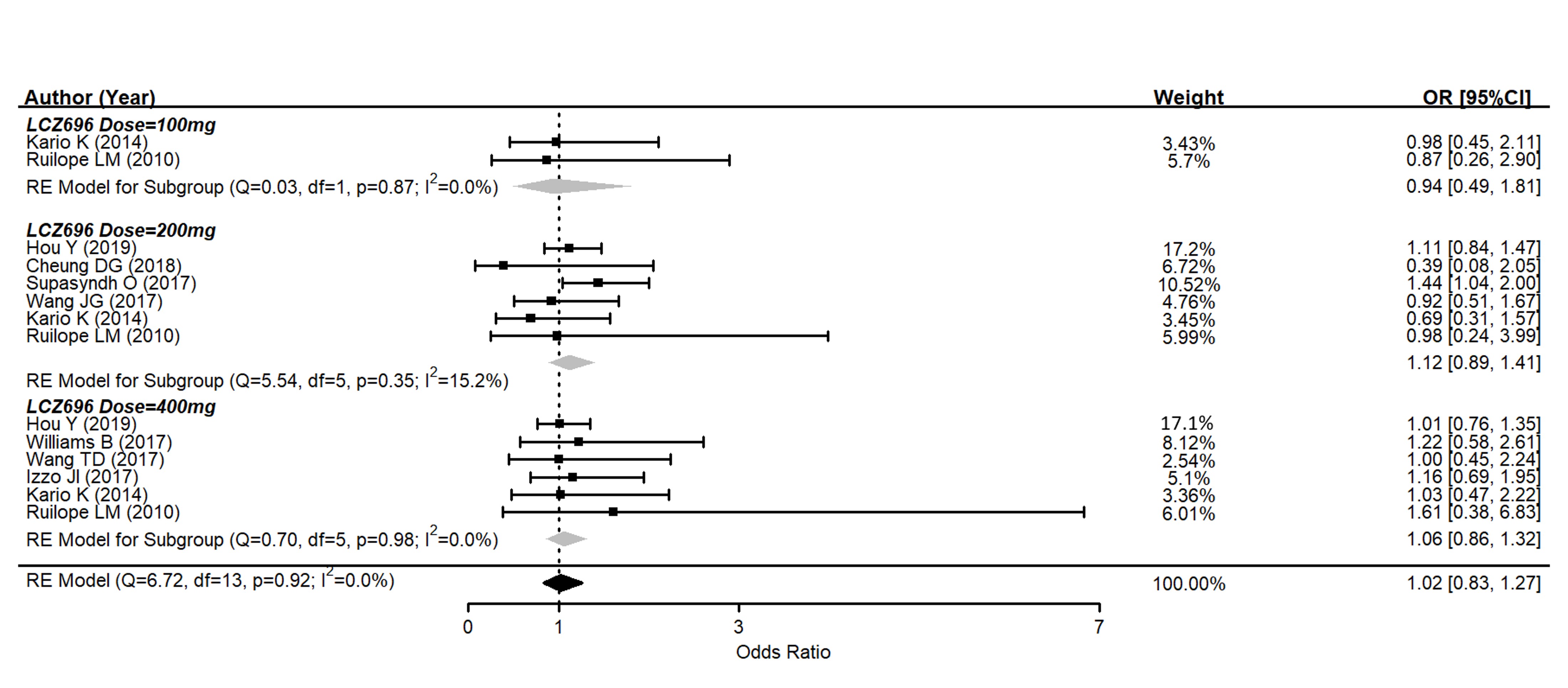
Figure 4. Forest plot of trial-defined adverse events. Comparisons between LCZ696 and a control group.
There were two cases of angioedema in the LCZ696 groups and one case in the olmesartan group, all of which were mild and resolved without hospitalization. Except for angioedema, there was one case of acute hepatitis that was attributed to LCZ696[8], but the patient recovered spontaneously. Drug discontinuation due to medical intolerance was comparable, at about 1% in each group.
6. Adjunctive Evaluations Concerning the Risk of Publication Bias and the Stability of Results
All studies had randomized controlled and double-blind trials, and all the prespecified outcomes were reported. The ten trials were parallel group, multicenter trials. Overall, the included studies were of high quality. The Egger’s test revealed that there was a publication bias that existed in the estimates of pooled effect size of msSBP, but not in other outcomes. (Figure 5)
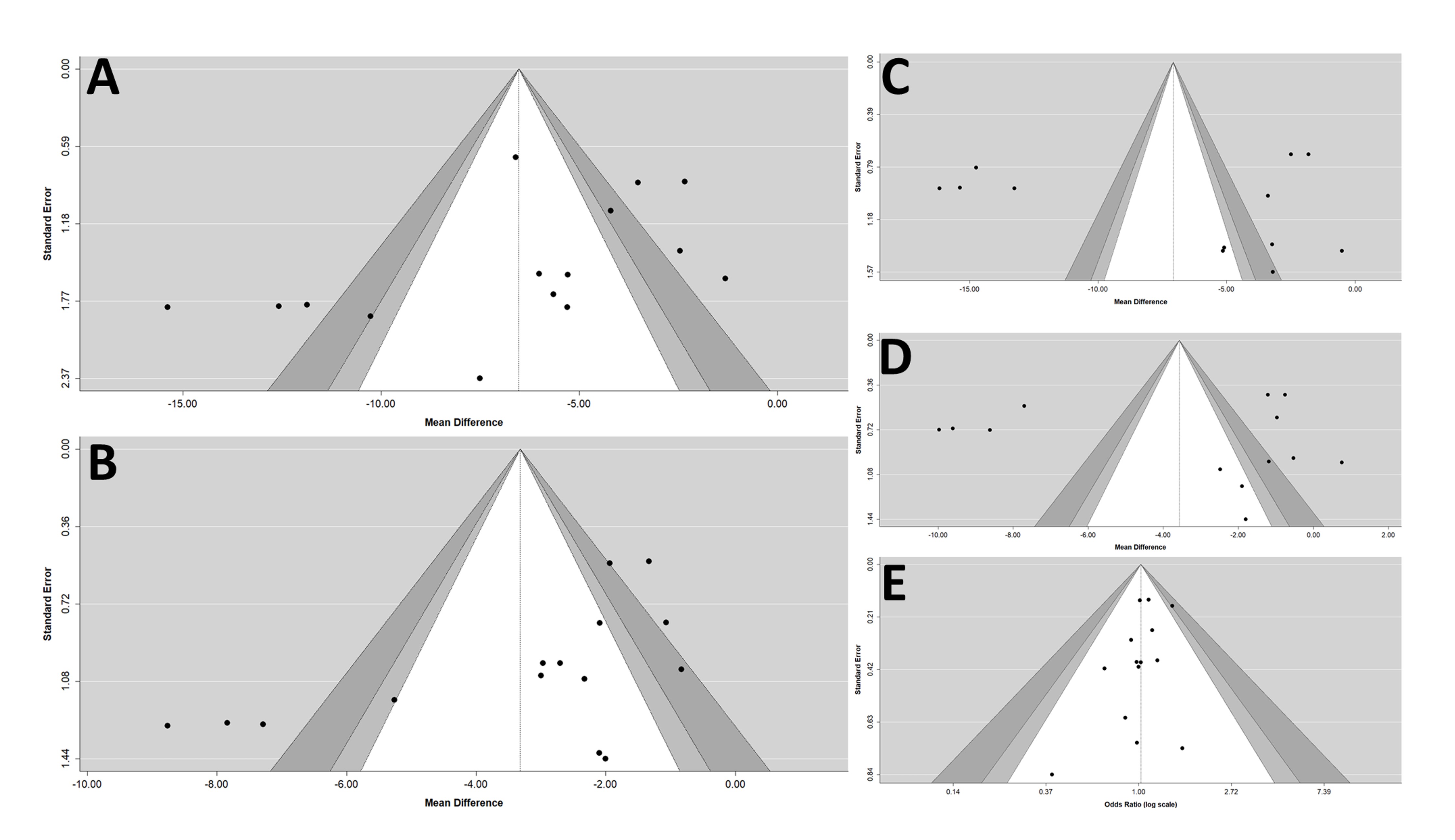
Figure 5. Funnel plots of (A) msSBP, (B) msDBP, (C) maSBP, (D) maDBP, and (E) adverse events. maSBP, mean ambulatory systolic blood pressure; maDBP, mean ambulatory diastolic blood pressure; msSBP, mean sitting systolic blood pressure; msDBP, mean sitting diastolic blood pressure.
References
- W.J. Elliott; Global burden of blood-pressure-related disease, 2001. Yearbook of Cardiology 2009, 2009, 16-18, 10.1016/s0145-4145(08)79402-4.
- Paul Muntner; Robert M. Carey; Samuel Gidding; Daniel W. Jones; Sandra J. Taler; Jackson T. Wright; Paul K. Whelton; Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Journal of the American College of Cardiology 2018, 71, 109-118, 10.1016/j.jacc.2017.10.073.
- Jeong Bae Park; Kazuomi Kario; Ji-Guang Wang; Systolic hypertension: an increasing clinical challenge in Asia. Hypertension Research 2014, 38, 227-236, 10.1038/hr.2014.169.
- John J.V. McMurray; Milton Packer; Akshay S. Desai; Jianjian Gong; Martin P. Lefkowitz; Adel R. Rizkala; Jean L. Rouleau; Victor C. Shi; Scott D. Solomon; Karl Swedberg; et al.Michael Zile Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. New England Journal of Medicine 2014, 371, 993-1004, 10.1056/nejmoa1409077.
- Deepak K. Gupta; Thomas J. Wang; Natriuretic Peptides and Cardiometabolic Health.. Circulation Journal 2015, 79, 1647-55, 10.1253/circj.CJ-15-0589.
- G.F. Mitchell; J.L. Izzo; Y. Lacourciere; Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension. results of the conduit hemodynamics of omipatrilat international research study. ACC Current Journal Review 2002, 11, 57-58, 10.1016/s1062-1458(02)00943-1.
- Luis Miguel Ruilope; Andrej Dukat; Michael Böhm; Yves Lacourcière; Jianjian Gong; Martin P Lefkowitz; Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. The Lancet 2010, 375, 1255-1266, 10.1016/s0140-6736(09)61966-8.
- Kazuomi Kario; Ningling Sun; Fu-Tien Chiang; Ouppatham Supasyndh; Sang Hong Baek; Akiko Inubushi-Molessa; Ying Zhang; Hiromi Gotou; Martin Lefkowitz; Jack Zhang; et al. Efficacy and Safety of LCZ696, a First-in-Class Angiotensin Receptor Neprilysin Inhibitor, in Asian Patients With Hypertension. Hypertension 2014, 63, 698-705, 10.1161/hypertensionaha.113.02002.
- Ji-Guang Wang; Kimihiko Yukisada; Antonio Sibulo; Kudsia Hafeez; Yan Jia; Jack Zhang; Efficacy and safety of sacubitril/valsartan (LCZ696) add-on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. Journal of Hypertension 2017, 35, 877-885, 10.1097/hjh.0000000000001219.
- Joseph L. Izzo; Dion H. Zappe; Yan Jia; Kudsia Hafeez; Jack Zhang; Efficacy and Safety of Crystalline Valsartan/Sacubitril (LCZ696) Compared With Placebo and Combinations of Free Valsartan and Sacubitril in Patients With Systolic Hypertension: The RATIO Study. Journal of Cardiovascular Pharmacology 2017, 69, 374-381, 10.1097/fjc.0000000000000485.
- Roland E. Schmieder; Frank Wagner; Michael Mayr; Christian Delles; Christian Ott; Christian Keicher; Maja Hrabak Paar; Tobias Heye; Solveig Aichner; Yasser Khder; et al.Denise YatesDiego AlbrechtThomas LangenickelPatrick FreyhardtRolf JankaJens Bremerich The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. European Heart Journal 2017, 38, 3308-3317, 10.1093/eurheartj/ehx525.
- Tzung-Dau Wang; Ru San Tan; Hae-Young Lee; Sang-Hyun Ihm; Moo-Yong Rhee; Brian Tomlinson; Parasar Pal; Fan Yang; Elizabeth Hirschhorn; Margaret F. Prescott; et al.Markus HinderThomas H. Langenickel Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT-proBNP in Salt-Sensitive Hypertension. Hypertension 2017, 69, 32-41, 10.1161/hypertensionaha.116.08484.
- Bryan Williams; John R. Cockcroft; Kazuomi Kario; Dion H. Zappe; Patrick C. Brunel; Qian Wang; Weinong Guo; Effects of Sacubitril/Valsartan Versus Olmesartan on Central Hemodynamics in the Elderly With Systolic Hypertension. Hypertension 2017, 69, 411-420, 10.1161/hypertensionaha.116.08556.
- Yong Huo; Weimin Li; Randy Webb; Li Zhao; Qian Wang; Weinong Guo; Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double‐blind, 8‐week study. The Journal of Clinical Hypertension 2018, 21, 67-76, 10.1111/jch.13437.
- Deanna G. Cheung; Diego Aizenberg; Vladimir Gorbunov; Kudsia Hafeez; Chien-Wei Chen; Jack Zhang; Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: A randomized, double-blind, 8-week study. The Journal of Clinical Hypertension 2018, 20, 150-158, 10.1111/jch.13153.
- Ouppatham Supasyndh; Jian’An Wang; Kudsia Hafeez; Ying Zhang; Hiromi Rakugi; Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Compared With Olmesartan in Elderly Asian Patients (≥65 Years) With Systolic Hypertension. American Journal of Hypertension 2017, 30, 1163-1169, 10.1093/ajh/hpx111.
- Liwen Ye; Jian Wang; Qingwei Chen; Xixi Yang; LCZ696, a promising novel agent in treating hypertension (a meta-analysis of randomized controlled trials). Oncotarget 2017, 8, 107991-108005, 10.18632/oncotarget.22442.
- Yang Zhao; Heng Yu; Xu Zhao; Ruixin Ma; NingYin Li; Jing Yu; The Effects of LCZ696 in Patients With Hypertension Compared With Angiotensin Receptor Blockers. Journal of Cardiovascular Pharmacology and Therapeutics 2017, 22, 447-457, 10.1177/1074248417693379.
- Renato De Vecchis; Silvia Soreca; Carmelina Ariano; Anti-Hypertensive Effect of Sacubitril/Valsartan: A Meta-Analysis of Randomized Controlled Trials. Cardiology Research 2019, 10, 24-33, 10.14740/cr813.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
833
Entry Collection:
Hypertension and Cardiovascular Diseases
Revision:
1 time
(View History)
Update Date:
09 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




