
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Philippe D'ABADIE | + 2510 word(s) | 2510 | 2021-07-08 11:11:06 | | | |
| 2 | Lindsay Dong | Meta information modification | 2510 | 2021-07-09 03:28:28 | | |
Video Upload Options
Inert microspheres, labeled with several radionuclides, have been developed during the last two decades for the intra-arterial treatment of liver tumors, generally called Selective Intrahepatic radiotherapy (SIRT). The aim is to embolize microspheres into the hepatic capillaries, accessible through the hepatic artery, to deliver high levels of local radiation to primary (such as hepatocarcinoma, HCC) or secondary (metastases from several primary cancers, e.g., colorectal, melanoma, neuro-endocrine tumors) liver tumors. Several types of microspheres were designed as medical devices, using different vehicles (glass, resin, poly-lactic acid) and labeled with different radionuclides, 90Y and 166Ho.
1. Introduction
Liver radioembolization (RE) is commonly used for the treatment of hepatocellular carcinoma and secondary liver malignancies. This treatment is performed by injection of radioactive microspheres in the liver artery after transarterial catheterization. Radioactive microspheres are trapped in the microvasculature (arterioles) of tumors and of the liver parenchyma. Unlike the liver parenchyma, where blood supply is almost obtained by the portal vein, liver tumors are preferentially vascularized by the liver arteries. This preferential perfusion allows us to achieve good targeting of hypervascularized tumors with a limited radiation of the non-tumoral liver [1]. The technique was initially developed using iodine-131 (131I) Lipiodol, a radiolabeled ethiodized oil [2]. Thereafter, radiolabeled microspheres have emerged using Yttrium-90 (90Y) and holmium-166 (166Ho). These radionuclides emit beta radiations of high energy, resulting in a high delivery of energy to the tumors (absorbed doses) in the range of 100 to 1000 Gy [3]. In comparison, the total tumor dose is limited to a maximum of 70 Gy in external beam radiotherapy to avoid liver damage [4].
Three types of microspheres are commercially available: 90Y-resin microspheres (Sir-Spheres®, Sirtex Medical Ltd., Sydney, Australia), 90Y-glass microspheres (Therasphere®, Boston Scientific, Boston, MA, USA) and 166Ho-poly-L-lactic acid (PLLA) microspheres (QuiremSpheres®, Quirem Medical B.V., Deventer, The Netherlands). Other microspheres have been used but did not reach the level of marketing authorization in Europe, e.g., 188Re-microspheres [5].
2. Preparations and Labeling (Sir-Spheres®, Therasphere®, QuiremSpheres®)
SIR-Spheres® are cation exchange resin microspheres labeled with 90Y phosphate. The resin is supplied as symmetrical microspheres ranging from 30 to 50 µm in diameter, made of sulphuric acid groups attached to a styrene divinylbenzene copolymer resin (Aminex 50W-X4, Bio-Rad, Hercules, CA, USA). First, stable yttrium oxide (III) (89Y) is activated in a neutron beam to its radioisotope 90Y according to the nuclear reaction 89Y (n,γ) 90Y. The radioactive yttria (Y2O3) is then dissolved in sulphuric acid. 90Y is adsorbed onto the resin matrix by adding newly formed 90Y sulphate solution to the aqueous slurry of microspheres. To immobilize and stably incorporate the 90Y into the lattice, the radionuclide is precipitated as an insoluble phosphate salt by adding a tri-sodium phosphate solution. Finally, the microspheres are washed with a phosphate buffer solution and resuspended with water for injection [6].
3. Radionuclide Properties and Clinical Applications
Table 1 summarizes the main physical characteristics of 90Y and 166Ho radionuclides.
| Labeled Microspheres | Half-Life | Beta Emission (E. Max) |
Range of Beta Radiation | Other Emissions | |
|---|---|---|---|---|---|
| Yttrium-90 (90Y) | Resin or glass | 2.7 days | 2.28 MeV | Mean: 2.5 mm (Max: 11 mm) | Positron (32 × 10−6) |
| Holmium-166 (166Ho) | Poly (L-Lactic acid) | 1.1 day | 1.77 MeV (49%), 1.86 MeV (50%) | Mean: 2.5 mm (Max: 8.7 mm) | Gamma (6.7%) |
4. Radioactive Microspheres Properties
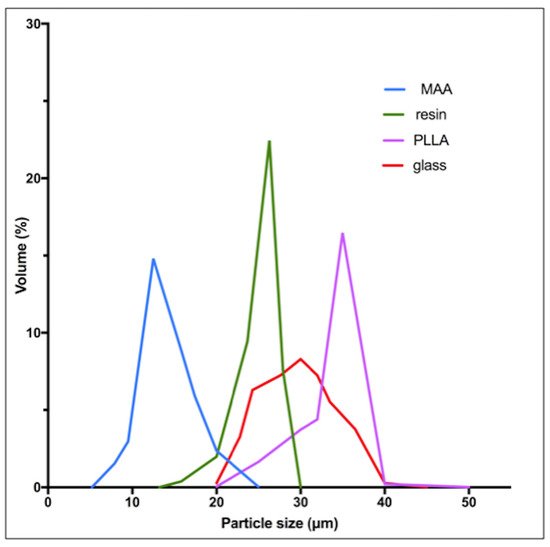
| Microspheres | Diameter (Mean) | Density | Approximative Number of Micro-Spheres Per GBq * | Activity Per Microsphere |
|---|---|---|---|---|
| 90Y-Resin | 32 μm | 1.6 g/mL | 13 × 106 | 50 Bq |
| 90Y-Glass | 25 μm | 3.3 g/mL | 0.4 × 106 | 2500 Bq |
| 166Ho-PLLA | 30 μm | 1.4 g/mL | 10 × 106 | 450 Bq |
5. Impact at a Microscopic Level-Microdosimetry
Due to their physical differences, these types of microspheres have a different distribution into the liver at the microscopic level, which results in major differences in local absorbed doses.
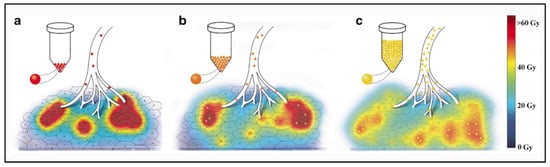
Contrarily to external radiotherapy, the absorbed dose distribution is heterogeneous in liver radioembolization resulting from the heterogeneous microsphere distribution at a microscopic level [22][23]. Moreover, due to their physical differences, the distribution of the absorbed dose into the liver is very different between the types of microspheres.
90Y-resin, 90Y-glass and 166Ho-PLLA microspheres have a similar diameter permitting us to similarly reach the microvasculature of tumors. The higher density of glass microspheres (Table 2) does not have a significant impact in the microsphere distribution compared to resin microspheres, as demonstrated by an experimental model of the hepatic vasculature [24].
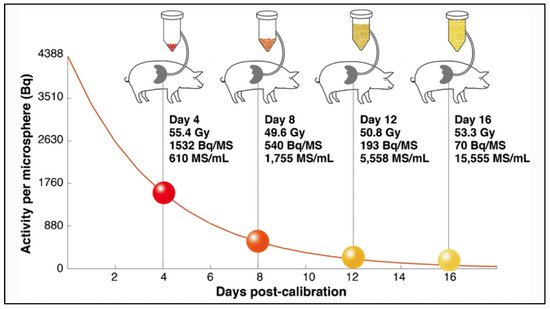
The main differences between microspheres are the specific activity per sphere and the number of injected microspheres during a treatment. Glass microspheres are especially highly radioactive (2500 Bq) and in limited number compared to others.
6. Impact at a Macroscopic Level-Clinical Effects
Physical characteristics of the radioactive microspheres explain also their different toxicity and efficacy profiles. Regarding PLLA microspheres (Quirem Spheres®), more data are needed to evaluate and compare its efficiency and toxicity.
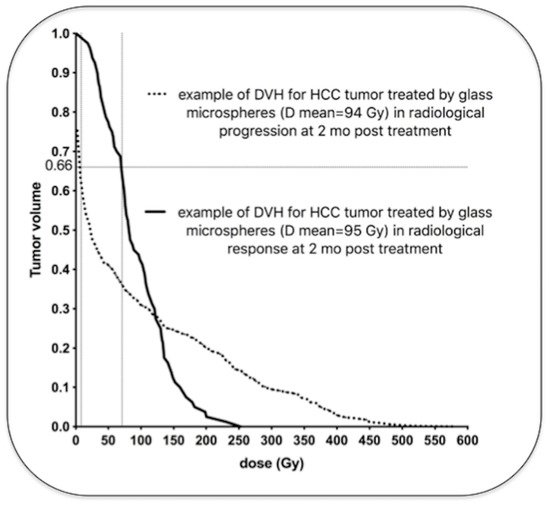
Regarding toxicity, an excessive irradiation of the healthy liver can induce a severe and potentially life-threatening complication: radioembolization induced liver disease (REILD) with an incidence rate inferior to 4% [26]. It is described by a sinusoidal obstruction syndrome and by a liver damage within 3 months after RE, in absence of tumor progression [27]. This complication tends to occur especially when a large volume of liver parenchyma is exposed to radiations. Other risk factors include a recent exposition to chemotherapy and underlying cirrhosis [28]. In external beam radiotherapy (EBRT), this complication occurs with whole liver doses of 30–35 Gy [29]. The tolerance is higher in RE, 40–50 Gy for resin microspheres and 90 Gy with glass microspheres [30][31][32]. A pre-clinical study in pigs with administration of PLLA microspheres demonstrates no toxicity with absorbed doses over 100 Gy. In human studies in phase 1 and 2, the whole liver absorbed dose was limited to 60 Gy and was not associated with any liver toxicity [33][34]. The homogeneity of the dose distribution explains the relative lower liver dose tolerability of external radiotherapy. To the contrary, a large heterogeneity of the dose distribution appears in RE, and a relative less proportion of liver parenchyma receives a toxic dose (i.e., 30–35 Gy). As described by Pasciak et al. [25], this liver dose heterogeneity is directly correlated to the number of microspheres injected in the liver. This characteristic could explain why the tolerable dose is significantly higher when a relatively small number of radioactive microspheres are injected into the liver (Table 2).
The 90Y beta range being sub-centimetric, the result is that, contrary to EBRT, the absorbed dose itself is highly heterogeneous. As a consequence, for an absorbed dose of 40 Gy, a sufficient fraction of lobules experienced sub-lethal dose in TARE and can repopulate the liver pool. Estimation of this fraction by Monte Carlo simulations explains the difference between the maximal safe absorbed dose between glass and resin spheres radioembolization, as well as EBRT [35].
References
- Lewandowski, R.J.; Salem, R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin. Interv. Radiol. 2006, 23, 64–72.
- Giammarile, F.; Bodei, L.; Chiesa, C.; Flux, G.; Forrer, F.; Kraeber-Bodere, F.; Brans, B.; Lambert, B.; Konijnenberg, M.; Borson-Chazot, F.; et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1393–1406.
- Kennedy, A.S.; Nutting, C.; Coldwell, D.; Gaiser, J.; Drachenberg, C. Pathologic response and microdosimetry of 90Y microspheres in man: Review of four explanted whole livers. Int. J. Radiat. Oncol. 2004, 60, 1552–1563.
- Kennedy, A. Radioembolization of hepatic tumors. J. Gastrointest. Oncol 2014, 5, 178–189.
- Lambert, B.; Bacher, K.; Defreyne, L. Rhenium-188 based radiopharmaceuticals for treatment of liver tumours. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 305–310.
- Gray, B.N. Polymer Based Radionuclide Containing Particulate Material. Patent No. WO2002034300A1, 2 May 2002.
- Westcott, M.A.; Coldwell, D.M.; Liu, D.M.; Zikria, J.F. The development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Adv. Radiat. Oncol. 2016, 1, 351–364.
- Day, D.; Ehrhardt, G. Glass Microspheres. U.S. Patent 4,789,501, 6 December 1986.
- Therasphere, Products Pecifications. Available online: (accessed on 13 May 2021).
- Nijsen, J.; van Steenbergen, M.; Kooijman, H.; Talsma, H.; Kroon-Batenburg, L.; van de Weert, M.; van Rijk, P.; de Witte, A.; Schip, A.V.H.; Hennink, W. Characterization of poly(l-lactic acid) microspheres loaded with holmium acetylacetonate. Biomaterials 2001, 22, 3073–3081.
- Nijsen, J.F.; Zonnenberg, B.A.; Woittiez, J.R.; Rook, D.W.; Swildens-van Woudenberg, I.A.; van Rijk, P.P.; van het Schip, A.D. Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: Effects of preparation and neutron activation techniques. Eur. J. Nucl. Med. 1999, 26, 699–704.
- Zielhuis, S.; Nijsen, J.; de Roos, R.; Krijger, G.; van Rijk, P.; Hennink, W.; Schip, A.V.H. Production of GMP-grade radioactive holmium loaded poly(l-lactic acid) microspheres for clinical application. Int. J. Pharm. 2006, 311, 69–74.
- Arranja, A.; Hennink, W.; Chassagne, C.; Denkova, A.; Nijsen, J. Preparation and characterization of inorganic radioactive holmium-166 microspheres for internal radionuclide therapy. Mater. Sci. Eng. C 2020, 106, 110244.
- QuiremSpheres. Instruction for Use. Available online: (accessed on 5 October 2020).
- Zhao, J.; Zhou, M.; Li, C. Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy. Cancer Nanotechnol. 2016, 7, 1–23.
- Brans, B.; Bodei, L.; Giammarile, F.; Linden, O.; Luster, M.; Oyen, W.J.G.; Tennvall, J. Clinical radionuclide therapy dosimetry: The quest for the "Holy Gray". Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 772–786.
- Sofou, S. Radionuclide carriers for targeting of cancer. Int. J. Nanomed. 2008, 3, 181–199.
- Vente, M.A.; Nijsen, J.F.; de Wit, T.C.; Seppenwoolde, J.H.; Krijger, G.C.; Seevinck, P.R.; Huisman, A.; Zonnenberg, B.A.; van den Ingh, T.S.; van het Schip, A.D. Clinical effects of transcatheter hepatic arterial embolization with holmium-166 poly (L-lactic acid) microspheres in healthy pigs. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1259–1271.
- Bakker, R.C.; de Roos, R.; Ververs, F.T.; Lam, M.G.; van der Lee, M.K.; Zonnenberg, B.A.; Krijger, G.C. Blood and urine analyses after radioembolization of liver malignancies with [166Ho]Ho-acetylacetonate-poly(l-lactic acid) microspheres. Nucl. Med. Biol. 2019, 71, 11–18.
- Bult, W. Holmium Microparticles for Intratumoral Radioablation. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2010.
- Gupta, T.; Virmani, S.; Neidt, T.M.; Szolc-Kowalska, B.; Sato, K.T.; Ryu, R.K.; Lewandowski, R.J.; Gates, V.L.; Woloschak, G.E.; Salem, R.; et al. MR tracking of iron-labeled glass radioembolization microspheres during transcatheter delivery to rabbit VX2 liver tumors: Feasibility study. Radiology 2008, 249, 845–854.
- Hogberg, J.; Rizell, M.; Hultborn, R.; Svensson, J.; Henrikson, O.; Molne, J.; Gjertsson, P.; Bernhardt, P. Increased absorbed liver dose in Selective Internal Radiation Therapy (SIRT) correlates with increased sphere-cluster frequency and absorbed dose inhomogeneity. EJNMMI Phys. 2015, 2, 10.
- Hogberg, J.; Rizell, M.; Hultborn, R.; Svensson, J.; Henrikson, O.; Molne, J.; Gjertsson, P.; Bernhardt, P. Heterogeneity of microsphere distribution in resected liver and tumour tissue following selective intrahepatic radiotherapy. EJNMMI Res. 2014, 4, 48.
- Caine, M.; McCafferty, M.S.; McGhee, S.; Garcia, P.; Mullett, W.M.; Zhang, X.; Hill, M.; Dreher, M.R.; Lewis, A.L. Impact of Yttrium-90 Microsphere Density, Flow Dynamics, and Administration Technique on Spatial Distribution: Analysis Using an In Vitro Model. J. Vasc. Interv. Radiol. 2017, 28, 260–268.e2.
- Pasciak, A.S.; Abiola, G.; Liddell, R.P.; Crookston, N.; Besharati, S.; Donahue, D.; Thompson, R.E.; Frey, E.; Anders, R.A.; Dreher, M.R.; et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 816–827.
- Riaz, A.; Awais, R.; Salem, R. Side effects of yttrium-90 radioembolization. Front. Oncol. 2014, 4, 198.
- Braat, A.J.; Smits, M.L.; Braat, M.N.; van den Hoven, A.F.; Prince, J.F.; de Jong, H.W.; van den Bosch, M.A.; Lam, M.G. 90Y Hepatic Radioembolization: An Update on Current Practice and Recent Developments. J. Nucl. Med. 2015, 56, 1079–1087.
- Gil-Alzugaray, B.; Chopitea, A.; Inarrairaegui, M.; Bilbao, J.I.; Rodriguez-Fraile, M.; Rodriguez, J.; Benito, A.; Dominguez, I.; D’Avola, D.; Herrero, J.I.; et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013, 57, 1078–1087.
- Koay, E.J.; Owen, D.; Das, P. Radiation-Induced Liver Disease and Modern Radiotherapy. Semin. Radiat. Oncol. 2018, 28, 321–331.
- Cremonesi, M.; Ferrari, M.; Bartolomei, M.; Orsi, F.; Bonomo, G.; Arico, D.; Mallia, A.; De Cicco, C.; Pedroli, G.; Paganelli, G. Radioembolisation with 90Y-microspheres: Dosimetric and radiobiological investigation for multi-cycle treatment. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2088–2096.
- Strigari, L.; Sciuto, R.; Rea, S.; Carpanese, L.; Pizzi, G.; Soriani, A.; Iaccarino, G.; Benassi, M.; Ettorre, G.M.; Maini, C.L. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: Radiobiologic considerations. J. Nucl. Med. 2010, 51, 1377–1385.
- Chiesa, C.; Mira, M.; Bhoori, S.; Bormolini, G.; Maccauro, M.; Spreafico, C.; Cascella, T.; Cavallo, A.; De Nile, M.C.; Mazzaglia, S.; et al. Radioembolization of hepatocarcinoma with 90Y glass microspheres: Treatment optimization using the dose-toxicity relationship. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3018–3032.
- Prince, J.F.; van den Bosch, M.; Nijsen, J.F.W.; Smits, M.L.J.; van den Hoven, A.F.; Nikolakopoulos, S.; Wessels, F.J.; Bruijnen, R.C.G.; Braat, M.; Zonnenberg, B.A.; et al. Efficacy of Radioembolization with 166Ho-Microspheres in Salvage Patients with Liver Metastases: A Phase 2 Study. J. Nucl. Med. 2018, 59, 582–588.
- Smits, M.L.; Nijsen, J.F.; van den Bosch, M.A.; Lam, M.G.; Vente, M.A.; Mali, W.P.; van Het Schip, A.D.; Zonnenberg, B.A. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): A phase 1, dose-escalation study. Lancet Oncol. 2012, 13, 1025–1034.
- Walrand, S.; Hesse, M.; Jamar, F.; Lhommel, R. A hepatic dose-toxicity model opening the way toward individualized radioembolization planning. J. Nucl. Med. 2014, 55, 1317–1322.
- Hermann, A.L.; Dieudonne, A.; Ronot, M.; Sanchez, M.; Pereira, H.; Chatellier, G.; Garin, E.; Castera, L.; Lebtahi, R.; Vilgrain, V.; et al. Relationship of Tumor Radiation-absorbed Dose to Survival and Response in Hepatocellular Carcinoma Treated with Transarterial Radioembolization with 90Y in the SARAH Study. Radiology 2020, 296, 673–684.
- Garin, E.; Lenoir, L.; Edeline, J.; Laffont, S.; Mesbah, H.; Poree, P.; Sulpice, L.; Boudjema, K.; Mesbah, M.; Guillygomarc’h, A.; et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: A new personalized promising concept. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1057–1068.
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29.
- Van den Hoven, A.F.; Rosenbaum, C.E.; Elias, S.G.; de Jong, H.W.; Koopman, M.; Verkooijen, H.M.; Alavi, A.; van den Bosch, M.A.; Lam, M.G. Insights into the Dose-Response Relationship of Radioembolization with Resin 90Y-Microspheres: A Prospective Cohort Study in Patients with Colorectal Cancer Liver Metastases. J. Nucl. Med. 2016, 57, 1014–1019.
- Willowson, K.P.; Hayes, A.R.; Chan, D.L.H.; Tapner, M.; Bernard, E.J.; Maher, R.; Pavlakis, N.; Clarke, S.J.; Bailey, D.L. Clinical and imaging-based prognostic factors in radioembolisation of liver metastases from colorectal cancer: A retrospective exploratory analysis. EJNMMI Res. 2017, 7, 46.
- Alsultan, A.A.; van Roekel, C.; Barentsz, M.W.; Smits, M.L.J.; Kunnen, B.; Koopman, M.; Bruijnen, R.C.G.; de Keizer, B.; Lam, M. Dose-response and dose-toxicity relationships for yttrium-90 glass radioembolization in patients with colorectal cancer liver metastases. J. Nucl. Med. 2021.
- Van Roekel, C.; Bastiaannet, R.; Smits, M.L.J.; Bruijnen, R.C.; Braat, A.; de Jong, H.; Elias, S.G.; Lam, M. Dose-Effect Relationships of 166Ho Radioembolization in Colorectal Cancer. J. Nucl. Med. 2021, 62, 272–279.
- D’Abadie, P.; Walrand, S.; Hesse, M.; Annet, L.; Borbath, I.; Van den Eynde, M.; Lhommel, R.; Jamar, F. Prediction of tumor response and patient outcome after radioembolization of hepatocellular carcinoma using 90Y-PET-computed tomography dosimetry. Nucl. Med. Commun. 2021.




