| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Oppedisano | + 1750 word(s) | 1750 | 2021-06-24 05:42:38 | | | |
| 2 | Vivi Li | Meta information modification | 1750 | 2021-07-07 03:57:14 | | |
Video Upload Options
Polyunsaturated fatty acids (n-3 PUFAs) are long-chain polyunsaturated fatty acids with 18, 20 or 22 carbon atoms, which have been found able to counteract cardiovascular diseases. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in particular, have been found to produce both vaso- and cardio-protective response via modulation of membrane phospholipids thereby improving cardiac mitochondrial functions and energy production. However, antioxidant properties of n-3 PUFAs, along with their anti-inflammatory effect in both blood vessels and cardiac cells, seem to exert beneficial effects in cardiovascular impairment. In fact, dietary supplementation with n-3 PUFAs has been demonstrated to reduce oxidative stress-related mitochondrial dysfunction and endothelial cell apoptosis, an effect occurring via an increased activity of endogenous antioxidant enzymes. On the other hand, n-3 PUFAs have been shown to counteract the release of pro-inflammatory cytokines in both vascular tissues and in the myocardium, thereby restoring vascular reactivity and myocardial performance.
1. Introduction
2. Antioxidant and Antinflammatory Properties of n-3 PUFAs
2.1. Mitochondrial Oxidative Stress and n-3 PUFAs
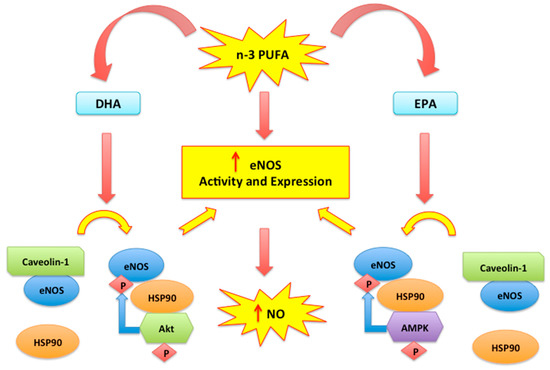
2.2. Nitric Oxide, Endothelial Dysfunction and n-3 PUFAs
2.3. Cell Membranes and Anti-Inflammatory Effects of n-3 PUFAs
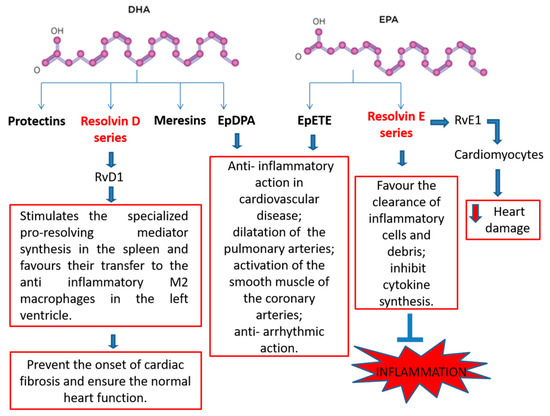
2.4. N-3 PUFA-Derived Mediators
References
- Sosnowska, B.; Penson, P.; Banach, M. The Role of Nutraceuticals in the Prevention of Cardiovascular Disease. Cardiovasc. Diagn. Ther. 2017, 7, S21–S31.
- Rivellese, A.A.; Ciciola, P.; Costabile, G.; Vetrani, C.; Vitale, M. The Possible Role of Nutraceuticals in the Prevention of Cardiovascular Disease. High Blood Press. Cardiovasc. Prev. 2019, 26, 101–111.
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18.
- Mollace, V.; Ragusa, S.; Sacco, I.; Muscoli, C.; Sculco, F.; Visalli, V.; Palma, E.; Muscoli, S.; Mondello, L.; Dugo, P.; et al. The protective effect of bergamot oil extract on lecitine-like oxyLDL receptor-1 expression in balloon injury-related neointima formation. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 120–129.
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. N-3 PUFAs Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 1, e867–e884.
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Gelejinse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of N-3 PUFAs Fatty Acid Supplement Use With Cardiovascular Disease Risks. JAMA Cardiol. 2018, 1, 225–234.
- Ganguly, R.; Hasanally, D.; Stamenkovic, A.; Maddaford, T.G.; Chaudhary, R.; Pierce, G.N.; Ravandi, A. Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol. Cell. Biochem. 2017.
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618.
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561.
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23.
- Cao, Y.; Lu, L.; Liang, J.; Liu, M.; Li, X.; Sun, R.; Zheng, Y.; Zhang, P. N-3 PUFAs Fatty Acids and Primary and Secondary Prevention of Cardiovascular Disease. Cell Biochem. Biophys. 2015, 72, 77–81.
- Chaddha, A.; Eagle, K.A. Cardiology Patient Page. N-3 PUFAs Fatty Acids and Heart Health. Circulation 2015, 132, e350ULATIe352.
- Kris-Etherton, P.M.; Fleming, J.A. Emerging nutrition science on fatty acids and cardiovascular disease: Nutritionists’ perspectives. Adv. Nutr. 2015, 6, 326S–337S.
- O′Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell. Cardiol. 2017, 103, 74–92.
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of n-3 PUFAs polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792.
- Tortosa-Caparros, E.; Navas-Carrillo, D.; Marin, F.; Orenes-Pinero, E. Anti-inflammatory Effects of Omega 3 and Omega 6 Polyunsaturated Fatty Acids in Cardiovascular Disease and Metabolic Syndrome. Crit. Rev. Food Sci. Nutr. 2017, 2, 3421–3429.
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin synthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252.
- Mollace, V.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F. Re-assessing the mechanism of action of n-3 PUFAs. Int. J. Cardiol. 2013, 170, S8–S11.
- Awada, M.; Soulage, C.O.; Mevnier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Estienne, M.; Peretti, N.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080.
- Wang, W.; Yang, H.; Johnson, D.; Gensler, C.; Decker, E.; Zhang, G. Chemistry and Biology of ω-3 PUFA Peroxidation-Derived Compounds. Prostaglandins Other Lipid Mediat. 2017, 132, 84–91.
- Yang, B.; Li, R.; Greenlief, C.M.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling Anti-Oxidative and Anti-Inflammatory Effects of Docosahexaenoic Acid and Its Lipid Peroxidation Product on Lipopolysaccharide-Stimulated BV-2 Microglial Cells. J. Neuroinflammation 2018, 9, 202.
- Mostowik, M.; Gajos, G.; Zalewski, J.; Nessler, J.; Undas, A. N-3 PUFAs polyunsaturated fatty acids increase plasma adiponectin to leptin ratio in stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 289–295.
- Roy, J.; Le Guennec, J.Y. Cardioprotective Effects of Omega 3 Fatty Acids: Origin of the Variability. J. Muscle Res. Cell Motil. 2017, 38, 25–30.
- Chrysohoou, C.; Metallinos, G.; Georgiopoulos, G.; Mendrinos, D.; Papanikolaou, A.; Magkas, N.; Pitsavos, C.; Vyssoulis, G.; Stefanadis, C.; Tousoulis, D. Short Term n-3 PUFAs Polyunsaturated Fatty Acid Supplementation Induces Favorable Changes in Right Ventricle Function and Diastolic Filling Pressure in Patients With Chronic Heart Failure; A Randomized Clinical Trial. Vasc. Pharmacol. 2016, 79, 43–50.
- Mozaffarian, D. Fish, n-3 Fatty Acids, and Cardiovascular Haemodynamics. J. Cardiovasc. Med. 2007, 8, S23–S26.
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species (Apex) 2019, 8, 312–322.
- Rodrigo, R.; Prieto, J.C.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: Molecular mechanisms and potential clinical applications. Clin. Sci. 2013, 124, 1–15.
- Herrera, E.A.; Farías, J.G.; González-Candia, A.; Short, S.E.; Carrasco-Pozo, C.; Castillo, R.L. Ω3 Supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar. Drugs 2015, 13, 838–860.
- Mozaffarian, D.; Wu, J.H.Y. N-3 PUFAs fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067.
- Cabo, J.; Alonso, R.; Mata, P. N-3 PUFAs fatty acids and blood pressure. Br. J. Nutr. 2012, 107, S195–S200.
- Gross, S.S.; Wolin, M.S. Nitric Oxide: Pathophysiological Mechanisms. Annu. Rev. Physiother. 1995, 57, 737–769.
- Moncada, S.; Higgs, E.A. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur. J. Clin. Investig. 1991, 21, 361–374.
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial Dysfunction as a Target for Prevention of Cardiovascular Disease. Diabetes Care 2009, 32, S314–S321.
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229.
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 4, 3294.
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022.
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 2007, 89, 169–177.
- Gousset-Dupont, A.; Robert, V.; Grynberg, A.; Lacour, B.; Tardivel, S. The effect of n-3 PUFA on eNOS activity and expression in Ea hy 926 cells. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 131–139.
- Wu, Y.; Zhang, C.; Dong, Y.; Wang, S.; Song, P.; Viollet, B.; Zou, M.H. Activation of the AMP-Activated Protein Kinase by Eicosapentaenoic Acid (EPA, 20:5 n-3) Improves Endothelial Function In Vivo. PLoS ONE 2012, 7, e35508.
- Zanetti, M.; Grillo, A.; Losurdo, P.; Panizon, E.; Mearelli, F.; Cattin, L.; Barazzoni, R.; Carretta, R. N-3 PUFAs Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. BioMed Res. Int. 2015, 2015, 791978.
- Lamoke, F.; Mazzone, V.; Persichini, T.; Maraschi, A.; Harris, M.B.; Venema, R.C.; Colasanti, M.; Gliozzi, M.; Muscoli, C.; Bartoli, M.; et al. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J. Neuroinflammation. 2015, 3, 12–84.
- Endo, J.; Arita, M. Cardioprotective mechanism of n-3 PUFAs polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27.
- Alzoubi, M.R.; Aldomi Al-Domi, H. Could n-3 PUFAs fatty acids a therapeutic treatment of the immune-metabolic consequence of intermittent hypoxia in obstructive sleep apnea? Diabetes Metab. Syndr. 2017, 11.
- Abdukeyum, G.G.; Owen, A.J.; Larkin, T.A.; McLennan, P.L. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. J. Clin. Med. 2016, 5, 32.
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory Activity of Polyunsaturated Fatty Acids in T-Cell Signaling. Prog. Lipid Res. 2010, 49, 250–261.
- Kakoti, B.B.; Hernandez-Ontiveros, D.G.; Kataki, M.S.; Shah, K.; Pathak, Y.; Panguluri, S.K. Resveratrol and N-3 PUFAs Fatty Acid: Its Implications in Cardiovascular Diseases. Front. Cardiovasc. Med. 2015, 2, 38.
- Jiang, J.; Li, K.; Wang, F.; Yang, B.; Fu, Y.; Zheng, J.; Li, D. Effect of Marine-Derived n-3 Polyunsaturated Fatty. PLoS ONE 2016, 25, e0147351.
- Recchiuti, A.; Serhan, C.N. Pro-Resolving Lipid Mediators (SPMs) and Their Actions in Regulating miRNA in Novel Resolution Circuits in Inflammation. Front. Immunol. 2012, 22, 298.
- Fredman, G.; Tabas, I. Boosting Inflammation Resolution in Atherosclerosis. Am. J. Pathol. 2017, 187, 1211–1221.
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101.
- Cash, J.L.; Norling, L.V.; Perretti, M. Resolution of inflammation: Targeting GPCRs that interact with lipids and peptides. Drug Discov. Today 2014, 19, 1186–1192.
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36.
- Herrera, B.S.; Hasturk, H.; Kantarci, A.; Freire, M.O.; Nguyen, O.; Kansal, S.; Van Dyke, T.E. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect. Immun. 2015, 83, 792–801.
- Keyes, K.T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J.R.; Gjorstrup, P.; Birnbaum, Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H153–H164.
- Kain, V.; Ingle, K.A.; Colas, R.A.; Dalli, J.; Prabhu, S.D.; Serhan, C.N.; Joshi, M.; Halade, G.V. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 2015, 84, 24–35.
- Duffield, J.S.; Hong, S.; Vaidya, V.S.; Lu, Y.; Fredman, G.; Serhan, C.N.; Bonventre, J.V. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006, 177, 5902–5911.
- Fischer, R.; Konkel, A.; Mehling, H.; Blossey, K.; Gapelyuk, A.; Wessel, N.; von Schacky, C.; Dechend, R.; Muller, D.N.; Rothe, M.; et al. Dietary n-3 PUFAs fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 2014, 55, 1150–1164.
- Gilbert, K.; Malick, M.; Madingou, N.; Touchette, C.; Bourque-Riel, V.; Tomaro, L.; Rousseau, G. Metabolites derived from n-3 PUFAs polyunsaturated fatty acids are important for cardioprotection. Eur. J. Pharmacol. 2015, 769, 147–153.